Abstract
Regenerative therapy in oral health care is limited by both the body's natural capacity for regeneration and the materials and methods currently available. Research on various aspects of regenerative therapy, such as tissue engineering and stem cell and gene therapy, has produced promising results. Compelling advances, ranging from the discovery and characterization of stem cell populations in oral tissue to the engineering and transplantation of whole tooth structures, could result in exciting new treatment methods for clinicians in the near future. In this review, we discuss the limitations of natural healing and regeneration of various tissues of the oral complex, including teeth, periodontium and salivary glands, and summarize current treatment methods for tissue damage as well as research advances in oral tissue regeneration.
Although tissues of the oral complex exhibit some regenerative capacity in response to disease, decay and trauma, damage to these tissues is frequently irreversible. Current clinical therapeutic approaches to replacing lost tissue aim at alleviating pain and restoring mechanical function. However, synthetic materials cannot replicate the physiological properties of the original tissue. Recent research has therefore focused on regeneration for the replacement of injured or dead tissue through such methods as tissue engineering, stem cell culture, embryology and developmental biology.1 In this paper, we review this work to update the clinician on available methods and research developments in oral tissue regeneration.
Promising Areas of Research
Tissue engineering has been the focus of tremendous research activity. There are 3 major classes of tissue engineering techniques: conductive, inductive and cell transplantation. Conductive techniques, such as guided tissue regeneration (GTR), use biomaterials passively to facilitate the growth or regeneration of existing tissue. Inductive techniques rely on the local delivery of growth factors, such as bone morphogenetic proteins and platelet-derived growth factor, to activate surviving cells and promote regeneration. Cell transplantation involves direct introduction of tissue previously manipulated in vitro.2 This involves taking existing tissue from the patient, isolating and multiplying the cells desired for regeneration and inserting them back into the tissue for reintegration.
Stem cell research offers a particularly effective potential method for cell transplantation and tissue regeneration.1 Conventional tissue transplantation solutions are limited by factors, such as insufficient donor tissue and graft rejection and failure. In contrast, stem cells may be able to regenerate new tissue and restore function. Stem cells are capable of renewing themselves through cell division and can be induced to become tissue- or organspecific.3 There are 2 types of stem cells: embryonic and adult (somatic). Embryonic stem cells are generally derived from eggs that have been fertilized in vitro and then donated for research purposes with the informed consent of the donor.3 Adult stem cells are undifferentiated cells found in a tissue or organ.
One major difference between embryonic and adult stem cells lies in the number and types of differentiated cells they can become. Embryonic stem cells are pluripotent, i.e., they can transform into any cell type found in the body. Adult stem cells are thought to be limited to differentiation into the cell types found in their tissue of origin; thus, they are multipotent.3 Most stem cells currently being tested for regenerative therapies are adult stem cells and are allogeneic (from a human donor other than the patient).4
Gene therapy, a facet of the inductive technique f tissue engineering, involves the use of transfer vectors, such as viruses or DNA, to insert or modify genes to treat disease. Recently, gene therapy techniques have been used in such areas of dentistry as pain management and bone or salivary gland repair.5
Challenges Surrounding Dental Tissue Regeneration
The reparative capacity of teeth in response to decay, wear and resorption is limited. Once lost, tooth enamel cannot regenerate, as the ameloblasts necessary for enamel formation lose their function after crown formation.6 Dentin and pulp, however, have some regenerative capacity depending on the state of vital pulp tissue.
The healing and regeneration of dental pulp is constrained, as it is encased in dentin and has a limited apical blood supply. The mechanism involved in the de-differentiation of pulp cells and the differentiation of associated progenitor cells has not yet been elucidated.7 Meanwhile, pathogens in the periodontium result in destruction of the tissue surrounding teeth via an inflammatory process that eventually culminates in tooth loss, which results in reduced oral and social function.8
Current treatments include partial or complete dentures, fixed bridges and implants. Dental implant therapy offers the most successful longterm rehabilitation, but it requires adequate preexisting bone structure.6 Whole tooth regeneration is intended to be a viable alternative to current treatment methods.
Current Periodontal Treatment Methods
Unlike dental tissue, periodontium is fully vascularized, permitting true and significant tissue regeneration. Bone or bone substitutes — autogenous grafts, allogeneic grafts, xenografts and alloplastic materials — can be placed in treated periodontal defects to promote regeneration (Fig. 1). In the treatment of intrabony defects, bone grafts increase clinical attachment level and reduce probing depth compared with conventional open flap debridement.9 No differences in clinical outcomes have been noted between different grafting materials.
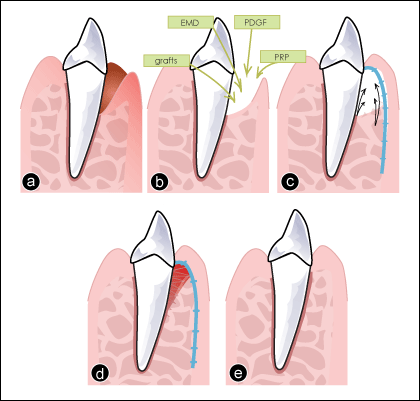 Figure 1: Current clinical approaches to regenerate periodontal defects. a) Tooth with a periodontal infrabony defect. b) Therapeutic options, such as bone grafts, enamel matrix derivative (EMD), platelet-derived growth factor (PDGF) or platelet-rich plasma (PRP), can be placed in the periodontal defect. c) A membrane (shown in blue) is inserted to guide tissue regeneration (black arrows). d) Integration of the grafted materials and healing (note: original defect now recolonized by cells and forming a new periodontal ligament). e) Final outcome after healing.
Figure 1: Current clinical approaches to regenerate periodontal defects. a) Tooth with a periodontal infrabony defect. b) Therapeutic options, such as bone grafts, enamel matrix derivative (EMD), platelet-derived growth factor (PDGF) or platelet-rich plasma (PRP), can be placed in the periodontal defect. c) A membrane (shown in blue) is inserted to guide tissue regeneration (black arrows). d) Integration of the grafted materials and healing (note: original defect now recolonized by cells and forming a new periodontal ligament). e) Final outcome after healing.
In guided tissue regeneration (GTR), a barrier membrane is used to promote the selective repopulation of the periodontal defect by cells derived from the periodontal ligament. Non-resorbable membranes are prone to exposure and infection and require a re-entry procedure to remove them. Resorbable membranes do not require re-entry, but are more often used in combination with bone grafts because of their non-supportive structure. GTR offers significant benefits in the management of intrabony defects and mandibular class II furcations, but the literature does not support the treatment of class III furcations.10 No differences have been reported between various types of barriers in terms of treatment of intrabony11 and furcation defects.10
Enamel matrix derivative (EMD) is a commercially available bioactive agent. Derived from unerupted porcine teeth and composed of amelogenins and enzyme components, EMD reproduces developmental mechanisms in which enamel matrix proteins play a critical role in stimulating cementogenesis.12 EMD increases attachment and reduces probing depth.13 These results have not been found with GTR, and GTR has been shown to increase gingival recession (0.4 mm) and post-operative complications.14 No advantages have been gained by combining EMD and GTR.13,14 Positive clinical outcomes can be maintained for 10 years after use of EMD alone or in combination with GTR.15
Bone morphogenetic proteins (BMPs) form a unique family within the transforming growth factor beta (TGF-β) proteins and have an essential role in regulation of bone formation, maintenance and repair.16 Two of these, BMP-2 and BMP-7 (osteogenic protein-1 [OP-1]), have been cloned and studied extensively for use in the oral cavity.
Human studies demonstrate the safety of BMP-2 for ridge preservation and sinus augmentation.17 Currently, recombinant human BMP-2 has Food and Drug Administration approval for use in sinus augmentation. As for periodontal indications, preclinical evaluations of BMP-2 demonstrate significantly enhanced bone and cementum formation, but not a functionally oriented periodontal ligament.18
OP-1 has been evaluated for periodontal wound healing and regeneration in surgically induced mandibular molar class II furcation defects in baboons.19 Sites receiving recombinant human OP-1 showed significant cementogenesis, including the presence of Sharpey fibers. OP-1 is also an active ingredient in Stryker Biotech bone graft material, for which osteoinductive and osteoconductive properties have been reported.20
Platelet-derived growth factor (PDGF) has been studied for periodontal regeneration in human clinical trials.13 Used with allogeneic bone grafts in the treatment of class II furcation21 and intrabony22 defects, PDGF has been shown to induce a substantial gain in attachment and a reduction in probing depth. A combination of PDGF with an alloplastic material, β-tricalcium phosphate (β-TCP), has also been tested without adverse effects23 and clinical outcomes were stable for 24 months.24 A commercial product containing PDGF and β-TCP, called GEM 21S, has been approved for the treatment of intrabony and furcation periodontal defects in the United States and Canada.
Platelet-rich plasma (PRP) is a platelet concentrate that contains a number of growth factors, including PDGF, TGF-β and IGF, which have been shown to have positive effects on periodontal wound healing.25 PRP can be prepared chairside, and safety issues are minimal for the patient as this material is autologous. In clinical trials, the use of PRP combined with several types of grafts for the treatment of intrabony defects has resulted in contradictory results ranging from significant enhancement of clinical attachment26-28 to no effect.29,30 This discrepancy in clinical outcomes may be partly due to differences in the methods used to obtain the PRP preparations, which could affect the content of platelets and inflammatory cytokines or lead to contamination of the platelet preparation with leucocytes and erythrocytes.31 The use of PRP does not result in adverse healing events following surgery, and some reports suggest that PRP may lead to more rapid healing, less postoperative pain and less membrane exposure.32,33
The Potential of Dental Stem Cells
The discovery and isolation of various types of dental (adult) stem cells has opened up opportunities for regeneration of tissues in the dentoalveolar complex, including pulp, periodontal tissue and perhaps, most important, whole teeth. Isolated populations include dental pulp stem cells (DPSC), stem cells from human exfoliated deciduous teeth (SHED), periodontal ligament stem cells (PDLSC), stem cells from the apical papilla (SCAP) and dental follicle stem cells (DFSC).34-36 The breadth of their potential for differentiation into mesenchymal tissues and their accessibility make them useful in numerous areas of stem-cell research. For example, human third molars are often extracted and discarded, but can be a suitable source of DPSC, PDLSC and SCAP if they are still undergoing root development37 (Fig. 2).
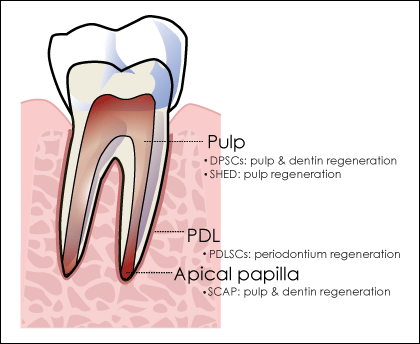 Figure 2: Areas of the tooth from which dental stem cells can be isolated and their potential uses in tissue regeneration.
Figure 2: Areas of the tooth from which dental stem cells can be isolated and their potential uses in tissue regeneration.
Note: DPSCs = dental pulp stem cells, SHED = stem cells from human exfoliated deciduous teeth, PDL=periodontal ligament, PDLSCs = periodontal ligament stem cells, SCAP = stem cells from the apical papilla.
Although none of these dental stem cells is able to differentiate to produce enamel, as ameloblasts are of ectodermal origin rather than mesenchymal, complete replication of the coronal shape and physiology of natural teeth may not be necessary. Clinically and functionally, the most important part of the tooth structure is the root and its supporting tissue. A crown alone cannot support mastication; however, a well-supported root structure with a conventional synthetic crown can provide an acceptable clinical outcome.38
Tooth Tissue Regeneration, Bioengineering and Future Prospects
Currently, 2 major approaches to tooth regeneration are being investigated.39,40 The first, which is based on tissue engineering, consists of seeding cells on scaffolding materials in vitro and subsequently implanting them. The second is based on reproducing the embryonic development of natural teeth through direct implantation of isolated engineered cells or biomaterials in vivo.
Young's group41 used the former technique to demonstrate the first successful bioengineering of recognizable tooth structures by breaking down porcine third molar buds into cell suspensions, seeding the suspensions onto biodegradable scaffolds and implanting them into rats. The resulting tooth-like structures comprised dentin and a pulp chamber, as well as Hertwig's sheath, cementoblasts and enamel; however, only 15% of the samples developed into properly organized tooth structures, and even these were smaller than naturally formed teeth.42
Meanwhile, other groups have used the technique of simulating embryonic development to create natural teeth or functional roots in mice and minipigs.40,43,44 Notably, fully formed tooth units including supporting periodontium have been generated and successfully integrated into jaw bone defects in mice.45 Furthermore, nondental mesenchymal stem cells, such as embryonic, neural and bone-marrow-derived stem cells, have been shown to produce properly formed teeth and periodontium via inductive signals from dental epithelium without the use of a scaffold.46
It remains to be determined whether such bioengineered teeth can achieve the masticatory function, biomechanical cooperation and sensory response of their naturally formed counterparts. Whole-tooth replacement, despite such promising recent advancements, is still far from being a readily applicable alternative to dental implant therapy. Localized cell-based tooth repair treatment would seem a more achievable short-term goal of regenerative dental therapy.47
Tissue Engineering and Gene Therapy in the Future of Periodontal Treatment
A tissue engineering approach to periodontal therapy has been proposed, whereby periodontal tissue would be constructed in the laboratory and then surgically implanted into defects.48,49 A promising technique involves harvesting stem cells from the PDL, culturing periodontal cell sheets in vitro and transplanting the tissue into periodontal defects. This has resulted in PDL tissue regeneration in animal models.50,51
Gene therapy can be used to facilitate extended local delivery of growth factors by transferring growth factor genes into the local cell population. The gene for platelet-derived growth factor (PDGF) has been successfully delivered into cementoblasts and other periodontal cell types.52-54 Animal studies have demonstrated that gene delivery of PDGF stimulates more cementoblast activity and regeneration than a single application of recombinant PDGF.51-53
Regenerating Lost Salivary Tissue
Xerostomia is the subjective perception of dry mouth resulting from a lack of saliva, often due to the hypofunction of salivary glands, specifically where epithelial acinar cells produce less fluid and proteins. It is a life-changing disorder that affects tasting, chewing and swallowing and can also lead to dental caries and periodontal and mucosal diseases, such as candidiasis and sialadenitis.55 Irreversible xerostomia is frequently caused by radiotherapy for head and neck cancers or by the autoimmune disorder Sjögren syndrome.
Therapeutic methods currently available focus on alleviating symptoms rather than restoring natural function. Salivary substitutes lack accuracy in mimicking the viscoelastic properties and surface rheology of saliva.56 Drug therapies, such as muscarinic receptor agonists (pilocarpine and cevimeline) and cholinesterase inhibitors are primarily dependant on neural stimulation of the surviving acinar cells to increase secretory capacity.57 Intraoral electrostimulation devices alleviate symptoms by enhancing the salivary reflex but do not restore tissue.58
Earlier studies51 have involved creating artificial salivary glands from isolated salivary cells and biodegradable scaffolds pre-coated with matrix proteins (Fig. 3). However, the available human salivary cell line (HSG) lacks tight junctions between cells, which are essential for unidirectional liquid–salt secretion.58 Although studies on salivary gland epithelial transplants from tissue digests have demonstrated potential, most reveal a limited capability of transplanted cells to replace salivary gland function.59 Isolated salivary cells rapidly dedifferentiate and undergo structural change as a result of loss of extracellular and intercellular communications, such as growth factors and intermittent neural stimulation.
 Figure 3: Experimental tissue- and bio-engineering approach to salivary gland regeneration. An artificial salivary gland (a tube) is constructed with a biodegradable substratum, matrix proteins and salivary graft cells. Ions (such as Na+ and Cl-) and water (H2O) move into the tube to form saliva. Below, human salivary acinar cells are cultured on a 3-dimensional (3D) gel or scaffold; these cells secrete proteins and growth factors. Both artificial salivary gland and 3D-gel culture of salivary cells can be transplanted into a patient to restore salivary gland function.
Figure 3: Experimental tissue- and bio-engineering approach to salivary gland regeneration. An artificial salivary gland (a tube) is constructed with a biodegradable substratum, matrix proteins and salivary graft cells. Ions (such as Na+ and Cl-) and water (H2O) move into the tube to form saliva. Below, human salivary acinar cells are cultured on a 3-dimensional (3D) gel or scaffold; these cells secrete proteins and growth factors. Both artificial salivary gland and 3D-gel culture of salivary cells can be transplanted into a patient to restore salivary gland function.
Animal models have been valuable in salivary gland regeneration studies. Research on mice has shown that ligation of the main excretory duct causes loss of acinar cells, but recovery from atrophy occurs after deligation.59 This has helped identify salivary progenitor cells of ductal origin that were positive for the stem-cell marker c-kit and that could restore gland functionality when transplanted into mice with radiation-induced glandular dysfunction.60 Although these cells did not transform into acinar cells, they were able to self-renew and regenerate the radiation-damaged salivary gland.56
Additional progenitor stem-cell markers were identified (Ascl3 and cytokeratin-5).61 However, salivary glands consist of numerous cells derived from different origins, making it challenging to select a specific stem cell that can differentiate into all types of cells. Once salivary progenitor cells are accurately localized and isolated in humans, they may potentially be used to treat salivary gland dysfunction in patients. Studies involving bone marrow-derived cells (BMDC) transplanted into irradiated mice indicate their potential to increase saliva production and regenerative activity, such as neovascularization.62,63 It has also been shown that BMDC and mesenchymal stromal cells produce immunomodulatory activity in mice with Sjögren-like disease.
Gene therapy is another potential method for repairing irradiation damage to salivary glands and treating Sjögren syndrome. This approach focuses on the delivery of a water-channel protein gene (aquaporin) to the surviving ductal epithelial cells using a recombinant adenovirus vector.64,65 In this method, once aquaporin is integrated into the membranes of ductal cells, they transform into fluid secreting cells, showing acinar-like function. Currently, a phase 1 clinical trial of an adenovirus containing the human aquaporin-1 gene, is underway in patients with parotid gland dysfunction due to irradiation treatment for head and neck cancer.
Conclusion
Significant discoveries and breakthroughs have occurred recently in the field of tissue regeneration in the oral complex. This progress provides a glimpse of future possibilities and has helped establish goals that may realistically be achieved in the coming decade. Adult stem cells isolated from teeth have the potential to develop into properly organized tooth components and whole structures. Engineered and transplanted periodontal tissue has been shown to increase regeneration in periodontal defects. Putative salivary progenitor stem cells are able to restore function in irradiation-damaged salivary glands. In addition to dental stem cells, other stem cells, such as mesenchymal and bone-marrowderived stem cells, have also proved useful for regenerating tissue in teeth, periodontium and salivary glands.
There are still major challenges to overcome before such revolutionary treatment options can become mainstream — namely their availability, biocompatibility, longevity, consistency and cost efficiency, which remain to be seen. However, with compelling advances in gene therapy, stem-cell research and tissue engineering, dentistry could come to provide exciting new treatment options in the near future.
THE AUTHORS
References
- Bulgin D, Hodzic E, Komljenovic-Blitva D. Advanced and prospective technologies for potential use in craniofacial tissues regeneration by stem cells and growth factors. J Craniofac Surg. 2011;22(1):342-8.
- Kaigler D, Mooney D. Tissue engineering's impact on dentistry. J Dent Educ. 2001;65(5):456-62.
- Stem cell basics. In Stem Cell Information. Bethesda, Md: National Institutes of Health, U.S. Department of Health and Human Services, 2009. Available: http://stemcells.nih.gov/info/basics/ (accessed 2012 Sept 18).
- Trounson A, Thakar RG, Lomax G, Gibbons D. Clinical trials for stem cell therapies. BMC Med. 2011;9:52.
- Baum BJ, Kok M, Tran SD, Yamano S. The impact of gene therapy on dentistry: a revisiting after six years. J Am Dent Assoc. 2002;133(1):35-44.
- Huang GT. Dental pulp and dentin tissue engineering and regeneration: advancement and challenge. Front Biosci (Elite Ed). 2011;3:788-800.
- Nakashima M, Iohara K, Zheng L. Gene therapy for dentin regeneration with bone morphogenetic proteins. Curr Gene Ther. 2006;6(5):551-60.
- Yen AH, Yelick PC. Dental tissue regeneration — a mini-review. Gerontology. 2011;57(1):85-94. Epub 2010 May 6.
- Reynolds MA, Aichelmann-Reidy ME, Branch-Mays GL, Gunsolley JC. The efficacy of bone replacement grafts in the treatment of periodontal osseous defects. A systematic review. Ann Periodontol. 2003;8(1):227-65.
- Sanz M, Giovannoli JL. Focus on furcation defects: guided tissue regeneration. Periodontol 2000. 2000;22(1):169-89.
- Murphy KG, Gunsolley JC. Guided tissue regeneration for the treatment of periodontal intrabony and furcation defects. A systematic review. Ann Periodontol. 2003;8(1):266-302.
- Hammarström L. Enamel matrix, cementum development and regeneration. J Clin Periodontol. 1997;24(9 Pt 2):658-68.
- Trombelli L, Farina R. Clinical outcomes with bioactive agents alone or in combination with grafting or guided tissue regeneration. J Clin Periodontol. 2008;35(8 Suppl):117-35.
- Sculean A, Schwarz F, Becker J, Brecx M. The application of an enamel matrix protein derivative (Emdogain) in regenerative periodontal therapy: a review. Med Princ Pract. 2007;16(3):167-80.
- Sculean A, Kiss A, Miliauskaite A, Schwarz F, Arweiler NB, Hannig M. Ten-year results following treatment of intra-bony defects with enamel matrix proteins and guided tissue regeneration. J Clin Periodontol. 2008;35(9):817-24. Epub 2008 Jul 21.
- Lee J, Stavropoulos A, Susin C, Wikesjö UM. Periodontal regeneration: focus on growth and differentiation factors. Dent Clin North Am. 2010;54(1):93-111.
- McAllister BS, Haghighat K. Bone augmentation techniques. J Periodontol. 2007;78(3):377-96.
- Ward BB, Brown SE, Krebsbach PH. Bioengineering strategies for regeneration of craniofacial bone: a review of emerging technologies. Oral Dis. 2010;16(8):709-16.
- Ripamonti U, Heliotis M, Rueger DC, Sampath TK. Induction of cementogenesis by recombinant human osteogenic protein-1 (hop-1/bmp-7) in the baboon (Papio ursinus). Arch Oral Biol. 1996;41(1):121-6.
- Chen FM, Zhang J, Zhang M, An Y, Chen F, Wu ZF. A review on endogenous regenerative technology in periodontal regenerative medicine. Biomaterials. 2010;31(31):7892-927. Epub 2010 Aug 4.
- Camelo M, Nevins ML, Schenk RK, Lynch SE, Nevins M. Periodontal regeneration in human Class II furcations using purified recombinant human platelet-derived growth factor-BB (rhPDGF-BB) with bone allograft. Int J Periodontics Restorative Dent. 2003;23(3):213-25.
- Nevins M, Camelo M, Nevins ML, Schenk RK, Lynch SE. Periodontal regeneration in humans using recombinant human platelet-derived growth factor-BB (rhPDGF-BB) and allogenic bone. J Periodontol. 2003;74(9):1282-92.
- Nevins M, Giannobile WV, McGuire MK, Kao RT, Mellonig JT, Hinrichs JE, et al. Platelet-derived growth factor stimulates bone fill and rate of attachment level gain: results of a large multicenter randomized controlled trial. J Periodontol. 2005;76(12):2205-15.
- McGuire MK, Kao RT, Nevins M, Lynch SE. rhPDGF-BB promotes healing of periodontal defects: 24-month clinical and radiographic observations. Int J Periodontics Restorative Dent. 2006;26(3):223-31.
- Okuda K, Kawase T, Momose M, Murata M, Saito Y, Suzuki H, et al. Platelet-rich plasma contains high levels of platelet-derived growth factor and transforming growth factor-beta and modulates the proliferation of periodontally related cells in vitro. J Periodontol. 2003;74(6):849-57.
- Hanna R, Trejo PM, Weltman RL. Treatment of intrabony defects with bovine-derived xenograft alone and in combination with platelet-rich plasma: a randomized clinical trial. J Periodontol. 2004;75(12):1668-77.
- Okuda K, Tai H, Tanabe K, Suzuki H, Sato T, Kawase T, et al. Platelet-rich plasma combined with a porous hydroxyapatite graft for the treatment of intrabony periodontal defects in humans: a comparative controlled clinical study. J Periodontol. 2005;76(6):890-8.
- Ouyang XY, Qiao J. Effect of platelet-rich plasma in the treatment of periodontal intrabony defects in humans. Chin Med J (Engl). 2006;119(18):1511-21.
- Demir B, Sengün D, Berberoğlu A. Clinical evaluation of platelet-rich plasma and bioactive glass in the treatment of intra-bony defects. J Clin Periodontol. 2007;34(8):709-15.
- Yassibag-Berkman Z, Tuncer O, Subasioglu T, Kantarci A. Combined use of platelet-rich plasma and bone grafting with or without guided tissue regeneration in the treatment of anterior interproximal defects. J Periodontol. 2007;78(5):801-9.
- Weibrich G, Kleis WK, Hafner G, Hitzler WE, Wagner W. Comparison of platelet, leukocyte, and growth factor levels in point-of-care platelet-enriched plasma, prepared using a modified Curasan kit, with preparations received from a local blood bank. Clin Oral Implants Res. 2003;14(3):357-62.
- Döri F, Huszár T, Nikolidakis D, Arweiler NB, Gera I, Sculean A. Effect of platelet-rich plasma on the healing of intrabony defects treated with an anorganic bovine bone mineral and expanded polytetrafluoroethylene membranes. J Periodontol. 2007;78(6):983-90.
- Papli R, Chen S. Surgical treatment of infrabony defects with autologous platelet concentrate or bioabsorbable barrier membrane: a prospective case series. J Periodontol. 2007;78(1):185-93.
- Casagrande L, Cordeiro MM, Nör SA, Nör JE. Dental pulp stem cells in regenerative dentistry. Odontology. 2011;99(1):1-7. Epub 2011 Jan 27.
- Mantesso A, Sharpe P. Dental stem cells for tooth regeneration and repair. Expert Opin Biol Ther. 2009;9(9):1143-54.
- Honda MJ, Imaizumi M, Tsuchiya S, Morsczeck C. Dental follicle stem cells and tissue engineering. J Oral Sci. 2010;52(4):541-52.
- Volponi AA, Pang Y, Sharpe PT. Stem cell-based biological tooth repair and regeneration. Trends Cell Biol. 2010;20(12):715-22. Epub 2010 Oct 28.
- Sonoyama W, Liu Y, Fang D, Yamaza T, Seo BM, Zhang C, et al. Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS One. 2006;1:e79.
- Nakahara T, Ide Y. Tooth regeneration: implications for the use of bioengineered organs in first-wave organ replacement. Hum Cell. 2007;20(3):63-70.
- Yen AH, Sharpe PT. Regeneration of teeth using stem cell-based tissue engineering. Expert Opin Biol Ther. 2006;6(1):9-16.
- Young CS, Terada S, Vacanti JP, Honda M, Bartlett JD, Yelick PC. Tissue engineering of complex tooth structures on biodegradable polymer scaffolds. J Dent Res. 2002;81(10):695-700.
- Young CS, Kim SW, Qin C, Baba O, Butler WT, Taylor RR, et al. Developmental analysis and computer modelling of bioengineered teeth. Arch Oral Biol. 2005;50(2):259-65.
- Ikeda E, Morita R, Nakao K, Ishida K, Nakamura T, Takano-Yamamoto T, et al. Fully functional bioengineered tooth replacement as an organ replacement therapy. Proc Natl Acad Sci U S A. 2009;106(32):13475-80. Epub 2009 Aug 3.
- Nakao K, Morita R, Saji Y, Ishida K, Tomita Y, Ogawa M, et al. The development of a bioengineered organ germ method. Nat Methods. 2007;4(3):227-30. Epub 2007 Feb 18.
- Oshima M, Mizuno M, Imamura A, Ogawa M, Yasukawa M, Yamazaki H, et al. Functional tooth regeneration using a bioengineered tooth unit as a mature organ replacement regenerative therapy. PLoS One. 2011;6(7):e21531. Epub 2011 Jul 12.
- Ohazama A, Modino SA, Miletich I, Sharpe PT. Stem-cell-based tissue engineering of murine teeth. J Dent Res. 2004;83(7):518-22.
- Ferreira CF, Magini RS, Sharpe PT. Biological tooth replacement and repair. J Oral Rehabil. 2007;34(12):933-9.
- Bartold PM, McCulloch CA, Narayanan AS, Pitaru S. Tissue engineering: a new paradigm for periodontal regeneration based on molecular and cell biology. Periodontol 2000. 2000;24:253-69.
- Lang H, Schüler N, Nolden R. Attachment formation following replantation of cultured cells into periodontal defects — a study in minipigs. J Dent Res. 1998;77(2):393-405.
- Iwata T, Yamato M, Tsuchioka H, Takagi R, Mukobata S, Washio K, et al. Periodontal regeneration with multi-layered periodontal ligament-derived cell sheets in a canine model. Biomaterials. 2009;30(14):2716-23. Epub 2009 Feb 7.
- Flores MG, Yashiro R, Washio K, Yamato M, Okano T, Ishikawa I. Periodontal ligament cell sheet promotes periodontal regeneration in athymic rats. J Clin Periodontol. 2008;35(12):1066-72.
- Anusaksathien O, Webb SA, Jin QM, Giannobile WV. Platelet-derived growth factor gene delivery stimulates ex vivo gingival repair. Tissue Eng. 2003;9(4):745-56.
- Chang PC, Cirelli JA, Jin Q, Seol YJ, Sugai JV, D'Silva NJ, et al. Adenovirus encoding human platelet-derived growth factor-B delivered to alveolar bone defects exhibits safety and biodistribution profiles favorable for clinical use. Hum Gene Ther. 2009;20(5):486-96.
- Jin Q, Anusaksathien O, Webb SA, Printz MA, Giannobile WV. Engineering of tooth-supporting structures by delivery of PDGF gene therapy vectors. Mol Ther. 2004;9(4):519-26.
- Meurman JH, Grönroos L. Oral and dental health care of oral cancer patients: hyposalivation, caries and infections. Oral Oncol. 2010;46(6):464-7. Epub 2010 Mar 21.
- Carpenter GH, Cotroneo E. Salivary gland regeneration. Front Oral Biol. 2010;14:107-28. Epub 2010 Apr 20.
- Strietzel FP, Lafaurie GI, Mendoza GR, Alajbeg I, Pejda S, Vuletić L, et al. Efficacy and safety of an intraoral electrostimulation device for xerostomia relief: a multicenter, randomized trial. Arthritis Rheum. 2011;63(1):180-90.
- Aframian DJ, Tran SD, Cukierman E, Yamada KM, Baum BJ. Absence of tight junction formation in an allogeneic graft cell line used for developing an engineered artificial salivary gland. Tissue Eng. 2002;8(5):871-8.
- Kagami H, Wang S, Hai B. Restoring the function of salivary glands. Oral Dis. 2008;14(1):15-24.
- Lombaert IM, Brunsting JF, Wierenga PK, Faber H, Stokman MA, Kok T, et al. Rescue of salivary gland function after stem cell transplantation in irradiated glands. PLoS One. 2008;3(4):e2063.
- Lombaert IM, Knox SM, Hoffman MP. Salivary gland progenitor cell biology provides a rationale for therapeutic salivary gland regeneration. Oral Dis. 2011;17(5):445-9. Epub 2011 Jan 11.
- Tran SD, Sumita Y, Khalili S. Bone marrow-derived cells: a potential approach for the treatment of xerostomia. Int J Biochem Cell Biol. 2011;43(1):5-9. Epub 2010 Oct 28.
- Khalili S, Liu Y, Kornete M, Roescher N, Kodama S, Peterson A, et al. Mesenchymal stromal cells improve salivary function and reduce lymphocytic infiltrates in mice with Sjögren's-like disease. PLoS One. 2012;7(6):e38615. Epub 2012 Jun 7.
- Baum BJ, Zheng C, Cotrim AP, McCullagh L, Goldsmith CM, Brahim JS, et al. Aquaporin-1 gene transfer to correct radiation-induced salivary hypofunction. Handb Exp Pharmacol. 2009(190):403-18.
- Baum BJ, Zheng C, Alevizos I, Cotrim AP, Liu S, McCullagh L, et al. Development of a gene transfer-based treatment for radiation-induced salivary hypofunction. Oral Oncol. 2010;46(1):4-8. Epub 2009 Nov 4.







