Abstract
Amelogenesis imperfecta (AI) is a hereditary disorder that causes developmental alterations in the structure of enamel. In addition, tooth sensitivity, missing or impacted teeth, taurodontism, altered dental esthetics and anterior open bite can also be associated with AI. This clinical report presents the diagnosis, treatment planning and prosthetic rehabilitation of a 19-year-old female patient with AI associated with a group of dental anomalies. Following clinical and radiographic examination, histologic evaluation of the teeth confirmed the diagnosis of rough pattern hypoplastic AI. The patient was rehabilitated with full-mouth zirconium oxide ceramic fixed bridges. Adaptation of the temporomandibular joints and masticatory muscles to the bridges was carefully observed over 3 years. At the end of this follow-up period, the patient was satisfied with the esthetics, function and phonation of her prostheses.
Amelogenesis imperfecta (AI) is a hereditary disorder, typically characterized by generalized enamel defects in both primary and permanent dentition.1,2 It might also be associated with morphologic or biochemical changes elsewhere in the body.1
AI may be an isolated finding or it may be part of a malformative syndrome, such as amelo-onycho-hypohidrotic syndrome, Morquio syndrome, Kohlschutter syndrome, tricho-dento-osseous syndrome, AI with taurodontism syndrome, oculo-dento-osseous dysplasia or epidermolysis bullosa hereditaria.3,4 Previous studies have also reported a rare syndrome associating AI with nephrocalcinosis, i.e., the precipitation of calcium salts in the kidney.5
Although the molecular basis of AI is not fully understood, in the past decade several mutations in the amelogenin (AMEL X) gene and enamelin gene have been identified in people with X-linked and autosomal forms of AI. No cases of mutation in the Y-chromosome amelogenin gene have been reported.5,6
The main types of AI are correlated with defects in the enamel synthesis process and have been classified into four broad categories based primarily on phenotype.7,8 There are also many subtypes. Class I is disturbance of the translation and secretion of an extracellular matrix (hypoplasia), class II is disturbance of the maturation of enamel (hypomaturation), class III is disturbance of the matrix mineralization process (hypocalcification) and class IV is hypomaturation-hypoplasia in combination with taurodontism.7,9,10
AI has been associated with several other dental anomalies, including dental or skeletal open bite, disturbances in eruption, congenitally missing teeth, pulpal calcification, hypercementosis, pathologic root and crown resorption, tooth sensitivity, poor dental esthetics, decreased vertical dimension and taurodontism.6,7,9 Poor oral hygiene and mouth breathing with associated gingivitis and gingival hyperplasia may adversely affect the oral health and the prognosis for prosthetic treatment of these patients.6 Thus, rehabilitation requires a multidisciplinary team consisting of a maxillofacial surgeon, a pediatric dentist, an orthodontist, a periodontist, an oral surgeon, a prosthodontist and a speech therapist.9,10
The multidisciplinary team should be in close collaboration in terms of planning the immediate, transitory and long-term phases of treatment. The orthodontist and pediatric dentist play important roles with regard to mixed dentition. They help guide the prosthodontist and oral surgeon, who might be needed to treat the permanent dentition. In addition, the role of the periodontist includes maintaining the health and structure of the masticatory system during and after AI treatment. The speech therapist plays an important role in restoring the system and in long-term management.10,11
The following clinical report describes the therapeutic planning and rehabilitative procedures experienced by a patient affected by AI.
Case Report
A 19-year-old woman was referred to Gazi University’s department of prosthodontics with the chief complaint of discolouration and unpleasant esthetics. Detailed dental, medical and social histories were obtained from the patient. Her general medical history was nonsignificant. She was born normally at term after an uneventful pregnancy. She was healthy and her general appearance was normal. A renal ultrasound scan was normal and showed no evidence of nephrocalcinosis. Laboratory findings, including serum electrolytes, calcium, phosphate, urea, creatinine, alkaline phosphatase and parathormone levels, were all normal; blood pressure was also normal. However, plasma calcium levels in her mother and father were slightly increased. Although the parents were not affected by AI, the patient’s brother was affected.
Clinical and radiographic examination of the patient revealed short crowns, occlusal wear with exposed dentin in the posterior areas and asymmetry of the gingival contours in the anterior maxillary teeth. Intraoral examination revealed yellow to yellowish-brown teeth with rough surfaces, conspicuous and irregular defects and a lack of contact points (Fig. 1). A deficiency in the mineral content of the enamel was evidenced by lack of radiographic opacity and a pathologic loss of enamel through wear and fracturing on both upper and lower teeth. On a panoramic radiograph, the thin enamel layer could not be distinguished from the underlying dentin (Fig. 2). The roots were normal in length and form and pulp chambers were regular in size. Root canal therapy and fillings were evident. In the left maxillary anterior region, the bite was open up to and including the first premolar.
Oral examination showed soft tissue to be within normal limits, and no periodontal pockets were detected. Gingival tissue was enlarged in the molar region. Molar occlusion appeared to be class I on the right side (Fig. 1b) and class III on the left (Fig. 1c). Based on these clinical findings, the diagnosis was generalized hypoplastic AI, with almost normal crown contours suggestive of mild, localized hypoplasia.
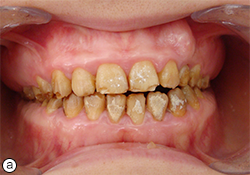
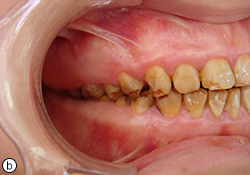
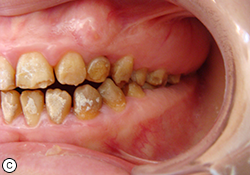
Fig. 1. Intraoral views before treatment show (a) discoloured teeth with rough surfaces and irregular defects, (b) class I molar occlusion on the right and (c) class III molar occlusion on the left.
In consultation with the patient, full maxillary and mandibular rehabilitation with zirconium oxide ceramic crowns extending to the second molars was considered to be the best therapeutic option. A diagnostic setup was prepared and informed consent was obtained from the patient. Under local anesthesia, teeth were prepared with circumferential shoulder margins of 1–1.2 mm and an occlusal reduction of 1.5–2 mm (Fig. 3). Provisional restorations were fabricated by the dental technician and cemented with noneugenol zinc oxide cement (TempBond NE, Kerr Corp, Orange, Calif).
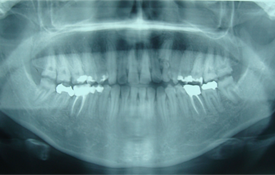 Fig. 2. Panoramic radiography before treatment reveals a very thin enamel layer, normal roots and pulp chambers, as well as earlier root canal therapy and fillings.
Fig. 2. Panoramic radiography before treatment reveals a very thin enamel layer, normal roots and pulp chambers, as well as earlier root canal therapy and fillings.
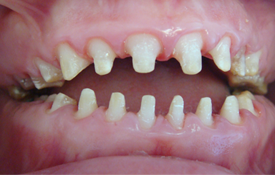 Fig. 3. Intraoral view after preparation of the teeth.
Fig. 3. Intraoral view after preparation of the teeth.
Definitive impressions of the maxillary and mandibular teeth and abutments were made with a polyether impression material (Impregum, 3M ESPE, St. Paul, Minn.). In the laboratory, a die model was prepared from type IV stone (ResinRock, Whip Mix Europe, Dortmund, Germany). Models were mounted using an arbitrary facebow (DentatusAED, Dentatus USA Ltd., New York, USA) and a centric relation record was obtained using polyether bite registration material (Ramitec, 3M ESPE) on a semi-adjustable articulator (Dentatus ARH, Dentatus USA Ltd.). The die model was scanned and the data were transmitted to a milling facility, where the frameworks were designed using White CAM 5.0 software (WhitePeaks Dental GmbH& Co. KG, Essen, Germany). Copings (Copran Zr®, WhitePeaks Dental GmbH& Co. KG) were manufactured with a thickness of 0.6 mm for the posterior and 0.4 mm for the anterior teeth (Fig. 4a,b). Porcelain veneering (Nobel Rondo zirconia dentine, Nobel Biocare AB, Gotenborg, Sweden) was completed, and the crowns were cleaned with ultrasound and 70% alcohol before cementation with a self-adhesive modified resin cement (Rely X Unicem, 3M ESPE AG, Seefeld, Germany) (Fig. 5). Occlusion was evaluated and adjusted intraorally to provide canine-guided consistent and regular contact between the crowns (Fig. 6a). The patient was instructed in the maintenance of interproximal gingival health using dental floss (Super Floss, Oral-B, Boston, Mass.). Routine panoramic radiographs were taken after treatment and annually during follow-up for 3 years (Fig. 6b).
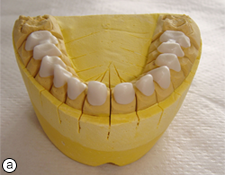
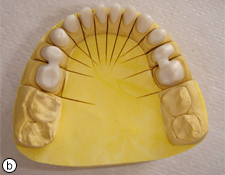
Figure 4. Zirconium oxide copings on the maxillary (a) and mandibular (b) master models.
 Fig. 5. Intraoral view during test positioning of the copings.
Fig. 5. Intraoral view during test positioning of the copings.
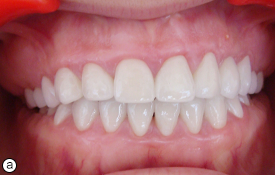
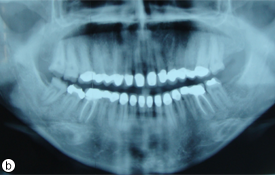
Fig. 6. Post-treatment intraoral (a) and radiographic (b) views showing restored teeth.

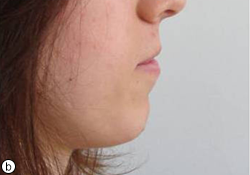
Fig. 7. The patient was very satisfied with the results in terms of both esthetics and function. Photographs taken after treatment: (a) from facial profile and (b) from right side.
The patient was followed at 3, 6 and 12 months and then annually with visual and radiographic examinations. During the first year, hygiene and long-term outcome were assessed. The patient acknowledged improved function and esthetics, and was pleased with the results (Fig. 7).
The maxillary and mandibular left third molars, which were decayed, were extracted after rehabilitation of the patient. Macroscopic examination with a stereomicroscope (Wild M3B, Heerbrugg, Switzerland) confirmed the diagnosis of thin hypoplastic AI. The extracted teeth were preserved for evaluation by scanning electron microscopy (SEM).
Histologic Evaluation
Molar teeth extracted from the patient were demineralized with 10% formic acid for 3 weeks, rinsed, dehydrated, and included in paraffin according to routine histological procedures. Teeth were then longitudinally cut, and 5-µm-thick sections were stained with hematoxylin-eosin stain. The analysis showed that there was no enamel layer. Although, no abnormality was present in the dentin, a thick primary dentin production had occurred on the coronal pulp chamber (Fig. 8).
SEM Evaluation
The tooth sections were coated with gold (SCD 050 Sputter Coater, Bal-Tec AG, Liechtenstein) and observed under SEM (JSM-5500, JEOL Ltd, Tokyo, Japan). While there were areas where the enamel had chipped off, a thin enamel layer with rough and nonuniform prismatic architecture was seen in some regions (Fig. 9).
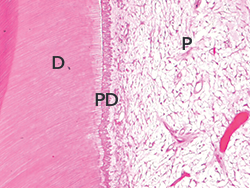 Fig. 8. Histologic analysis of a tooth affected by AI revealed areas with no enamel layer and thick primer dentin (Hematoxylin-eosin stain, original magnification x100) D=dentin, PD= primer dentin, P=pulp.
Fig. 8. Histologic analysis of a tooth affected by AI revealed areas with no enamel layer and thick primer dentin (Hematoxylin-eosin stain, original magnification x100) D=dentin, PD= primer dentin, P=pulp.
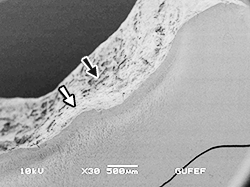 Fig. 9. Scanning electron micrograph of the same tooth shows areas with irregular and thin enamel layer (white arrow) and areas without enamel (black arrow). (Original magnification X30, bar = 500 µm).
Fig. 9. Scanning electron micrograph of the same tooth shows areas with irregular and thin enamel layer (white arrow) and areas without enamel (black arrow). (Original magnification X30, bar = 500 µm).
Discussion
Zirconium oxide-based restorative materials have excellent mechanical properties and low bacterial adhesion and they are biocompatible. This allows several applications in restorative dentistry, one of which is as a core material for ceramic crowns and fixed partial dentures.12,13 In addition, studies have reported that zirconium oxide restorations represent promising prosthetic treatments for posterior regions.14,15 The present case report describes the prosthetic rehabilitation of an adolescent patient with AI. The maxillary and mandibular right and left third molars were fully erupted; therefore, the permanent restorative material, zirconium oxide ceramic, was selected as a suitable replacement for the defective structures because of the attractive mechanical properties described above.
Previous studies have reported several methods for determining type of AI using combinations of clinical, radiographic, histologic and genetic criteria.16 Histologic evaluation using staining and SEM confirmed the diagnosis of AI. Although, SEM revealed a thin enamel layer with irregular structure, a thick layer of primary dentin had formed around the coronal pulp to resist traumatic forces.
Patients with this anomaly often seek treatment because of an unpleasant appearance, impaired mastication and social embarrasment.5 Lack of uniformity of the occclusal plane, congenitally missing teeth, tooth sensitivity, poor dental esthetics and decreased vertical dimension may create prosthodontic challenges.5
In the present case, the main complaints were tooth discolouration and generalized sensitivity. The patient was dissatisfied with her dental appearance and concerned about the long-term condition of her teeth. The aim was to satisfy her desire for esthetic improvement and not primarily to change her occlusal scheme. Therefore, full-mouth zirconium oxide ceramic rehabilitation of the patient was provided without changing the occlusal vertical dimension. The occlusion was adjusted according to the patient’s centric relation, as this was the only reproducible position.16 Canine-guided occlusion was provided, as the maxillary and mandibular canines came into contact on the working side while the posterior teeth were disoccluded.
After prosthetic rehabilitation, both facial appearance and occlusion were improved. Long-term dental management consisted of regular clinical and radiographic reviews at 3, 6, 12, 24 and 36 months. Function, phonation and esthetic expectations of the patient were met. In the radiologic and clinical examination, no problem was seen in soft tissue or in maintenance of the restorations. Function, parafunction, aging and stress fatigue affect the longevity of restorations in the oral environment.17 Therefore; continuous follow-up of at least 5 years will be needed to assess the success of such restorations in the oral cavity.18
Conclusion
In the presented case report, esthetic and functional rehabilitation of hypoplastic AI was performed with the use of zirconium oxide ceramic restorations. The patient’s functional and esthetic expectations were achieved and no problem was detected at the 3rd annual clinical visit.
THE AUTHORS
References
- Ramos AL, Pascotto RC, Iwaki Filho L, Hayacibara RM, Boselli G. Interdisciplinary treatment for a patient with open-bite malocclusion and amelogenesis imperfecta. Am J Orthod Dentofacial Orthop. 2011;139(4 Suppl):S145-53.
- El-Sayed W, Shore RC, Parry DA, Inglehearn CF, Mighell AJ. Hypomaturation amelogenesis imperfecta due to WDR72 mutations: a novel mutation and ultrastructural analyses of deciduous teeth. Cells Tissues Organs. 2011;194(1):60-6. Epub 2010 Dec 29.
- Martelli-Júnior H, dos Santos Neto PE, de Aquino SN, de Oliveira Santos CC, Borges SP, Oliveira EA, et al. Amelogenesis imperfecta and nephrocalcinosis syndrome: a case report and review of the literature. Nephron Physiol. 2011;118(3):62-5. Epub 2011 Jan 7.
- Santos MC, Hart PS, Ramaswami M, Kanno CM, Hart TC, Line SR. Exclusion of known gene for enamel development in two Brazilian families with amelogenesis imperfecta. Head Face Med. 2007;3:8.
- Paula LM, Melo NS, Silva Guerra EN, Mestrinho DH, Acevedo AC. Case report of a rare syndrome associating amelogenesis imperfecta and nephrocalcinosis in a consanguineous family. Arch Oral Biol. 2005;50(2):237-42.
- Hart TC, Hart PS, Gorry MC, Michalec MD, Ryu OH, Uygur C, et al. Novel ENAM mutation responsible for autosomal recessive amelogenesis imperfecta and localised enamel defects. J Med Genet. 2003;40(12):900-6.
- Urzúa B, Ortega-Pinto A, Morales-Bozo I, Rojas-Alcayaga G, Cifuentes V. Defining a new candidate gene for amelogenesis imperfecta: from molecular genetics to biochemistry. Biochem Genet. 2011;49(1-2):104-21. Epub 2010 Dec 3.
- Lindemeyer RG, Gibson CW, Wright TJ. Amelogenesis imperfecta due to a mutation of the enamelin gene: clinical case with genotype-phenotype correlations. Pediatr Dent. 2010;32(1):56-60.
- Gisler V, Enkling N, Zix J, Kim K, Kellerhoff NM, Mericske-Stern R. A multidisciplinary approach to the functional and esthetic rehabilitation of amelogenesis imperfecta and open bite deformity: a case report. J Esthet Restor Dent. 2010;22(5):282-93.
- Pires Dos Santos AP, Cabral CM, Moliterno LF, Oliveira BH. Amelogenesis imperfecta: report of a successful transitional treatment in the mixed dentition. J Dent Child (Chic). 2008;75(2):201-6.
- Arkutu N, Gadhia K, McDonald S, Malik K, Currie L. Amelogenesis imperfecta: the orthodontic perspective. Br Dent J. 2012;212(10):485-9.
- Raigrodski AJ, Chiche GJ, Potiket N, Hochstedler JL, Mohamed SE, Billiot S, et al. The efficacy of posterior three-unit zirconium-oxide-based ceramic fixed partial dental prostheses: a prospective clinical pilot study. J Prosthet Dent. 2006;96(4):237-44.
- Palacios RP, Johnson GH, Phillips KM, Raigrodski AJ. Retention of zirconium oxide ceramic crowns with three types of cement. J Prosthet Dent. 2006;96(2):104-14.
- Sorrentino R, De Simone G, Tetè S, Russo S, Zarone F. Five-year prospective clinical study of posterior three-unit zirconia-based fixed dental prostheses. Clin Oral Investig. 2012;16(3):977-85. Epub 2011 Jun 11.
- Peláez J, Cogolludo PG, Serrano B, Lozano JF, Suárez MJ. A prospective evaluation of zirconia posterior fixed dental prostheses: three-year clinical results. J Prosthet Dent. 2012;107(6):373-9.
- Chan KH, Ho EH, Botelho MG, Pow EH. Rehabilitation of amelogenesis imperfecta using a reorganized approach: a case report. Quintessence Int. 2011;42(5):385-91.
- Drummond JL. Ceramic behavior under different environmental and loading conditions. In: Eliades G, Eliades T, Brantley WA, Watts DC, editors. Dental Materials In Vivo: Aging and Related Phenomena. Chicago: Quintessence; 2003. p. 35-45.
- Wolfart S, Eschbach S, Scherrer S, Kern M. Clinical outcome of three-unit lithium-disilicate glass-ceramic fixed dental prostheses: up to 8 years results. Dent Mater. 2009;25(9):e63-71. Epub 2009 Jun 11.




