View Letters related to this article
Abstract
Proper management of an oral mucosal lesion begins with diagnosis, and the gold standard for diagnosing disease, oral or otherwise, is tissue biopsy. The oral environment, which is moist and confined, poses challenges for collecting a viable tissue sample that will be suitable for diagnosis. These challenges are further compounded by the myriad of biopsy techniques and devices now available. In addition, certain oral subsites are subject to diagnostic pitfalls that necessitate modifications of technique. This article provides an overview of the oral soft-tissue biopsy and highlights some potential pitfalls.
Biopsy is the removal of a tissue sample from a living body with the objective of providing the pathologist with a representative, viable specimen for histopathologic interpretation and diagnosis.1 This approach is used for all tissues of the body, including those of the oral cavity, where a wide spectrum of disease processes may present. The dental clinician should be aware of the various biopsy techniques that are available for the oral tissues, as well as the challenges specific to these tissues.
Types of Oral Lesions
The oral cavity is lined with stratified squamous epithelium overlying mesenchymal tissues. Benign and malignant neoplasms may originate from any of these tissues. Lesions of epithelial origin are typically white or red. Hyperplasia of the epithelium and accumulation of keratin produce a white lesion (Fig. 1), whereas epithelial atrophy allows greater visualization of the underlying vasculature, which leads to a red appearance. The epithelial integrity may be disrupted (a process known as ulceration), and resultant tissue proliferation may be exuberant, producing a papillary or verruciform configuration. Representative biopsy of an epithelial lesion must include the full epithelial thickness with some supporting connective tissue to allow assessment for invasive carcinoma but also to provide physical support for the specimen. There are no strict size criteria, but tiny samples impede processing and interpretation of the specimen.1 For a thin plaque, a sample only a few millimetres deep will typically suffice, and there is no need to extend deep into the connective tissues or musculature. However, a sample of greater depth will be needed if there is exuberant epithelial thickening due to epithelial hyperplasia, elongation of the rete ridges or accumulation of keratin, as may be the case in verrucous carcinoma or verrucous hyperplasia.
Lesions of mesenchymal origin arise below the epithelium and appear as dome-shaped swellings (Fig. 2). The overlying epithelium is often intact, but its coloration reflects the contents of the lesion: red–purple if vascular, blue if mucinous or yellow if adipose, lymphoid or neural. The representative biopsy sample must be of sufficient depth, but the appropriate depth varies from one lesion to another, depending on the thickness and location of the mass.
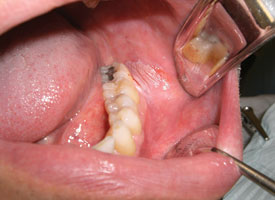 Figure 1: Thick, homogenous leukoplakia affecting the posterior buccal mucosa is characterized by hyperplasia of the epithelium and accumulation of keratin, producing a white lesion. Histologic assessment showed epithelial hyperplasia, hyperkeratosis and mild dysplasia.
Figure 1: Thick, homogenous leukoplakia affecting the posterior buccal mucosa is characterized by hyperplasia of the epithelium and accumulation of keratin, producing a white lesion. Histologic assessment showed epithelial hyperplasia, hyperkeratosis and mild dysplasia.
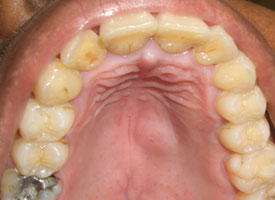 Figure 2: Benign salivary gland neoplasm (pleomorphic adenoma) presenting as a dome-shaped submucosal palatal swelling with a surface of intact pink mucosa.
Figure 2: Benign salivary gland neoplasm (pleomorphic adenoma) presenting as a dome-shaped submucosal palatal swelling with a surface of intact pink mucosa.
Preliminary Examination of the Lesion
A comprehensive dental examination will include both visual and tactile assessment of the oral soft tissues. Once an oral lesion has been identified, the clinician should undertake a standard protocol, beginning with eliciting and documenting the pertinent history, including duration, any antecedent event, symptoms and changes in appearance, as well as prior diagnostic and therapeutic measures. The location, size, colour, and consistency or texture of the lesion should be documented, which may be facilitated by photography. The differential diagnosis will guide management decisions, including the decision to obtain a biopsy sample. Referral to a clinician with expertise in the diagnosis and management of oral disease is always a possibility.
Indications for Biopsy
Biopsy is essential if there is any clinical suspicion of malignancy, such as an enlarging mass, chronic ulceration, tissue friability, induration on palpation or persistence of mucosal changes despite removal of local irritants.2-4 New or enlarging pigmented lesions, especially those with an irregular border and nonhomogenous coloration, should be biopsied to exclude mucosal melanoma. Entities that appear to be clinically benign or reactive (e.g., pyogenic granuloma or mucocele) may be excised for esthetic or functional reasons, but the tissues should be submitted for histologic analysis to confirm the clinical impression. Lichen planus, mucous membrane pemphigoid, pemphigus vulgaris and other immune-mediated disorders may present with widespread mucosal erythema and ulceration, and biopsy is essential for definitive diagnosis.
Contraindications for Biopsy
Significant hemorrhage may accompany biopsy of a vascular lesion, and caution should therefore be exercised in the biopsy of any lesion with red, purple or blue coloration or with blanching or pulsation on palpation. Location of the lesion in an esthetic region (e.g., vermilion border of the lip) is not a strict contraindication, but referral to a specialist should be considered in such cases. Similarly, certain oral subsites, such as the floor of the mouth, may be challenging to access, difficult to provide hemostasis and risks damage to anatomic structures (e.g., submandibular duct). It is unwise to proceed if one is uncomfortable with either the surgical procedure or the prospect of relaying devastating results to the patient.
Standard biopsy procedure may need to be modified and medical clearance obtained in the case of medically compromised patients, including those with severe or poorly controlled systemic diseases such as coronary artery disease, renal or hepatic impairment, and various endocrinopathies and immune-compromised states. Clearance from the primary medical caregiver and baseline serologic studies should be considered for patients with significant risk of hemorrhage due to underlying bleeding diatheses related to anticoagulant therapy, thrombocytopenia or any of various inherited coagulopathies (e.g., hemophilia or Von Willebrand disease). Patients undergoing bisphosphonate therapy or radiotherapy may be predisposed to osteonecrosis if the biopsy procedure exposes bone.
Biopsy Techniques
Scalpel biopsy, for both incisional and excisional procedures, is the most common technique and generally produces the most satisfactory samples.
Incisional Biopsy
Incisional biopsy provides a representative sample of tissue for diagnostic purposes. It is the method of choice when the differential diagnosis includes malignancy. Its accuracy is relative, since by nature it does not allow study of the entire lesion. Should the clinician be uncertain about the most appropriate site from which to obtain the biopsy sample, referral to a specialist is necessary.5
The clinician should attempt to sample the tissue that has been most severely and significantly affected. In cases of epithelial dysplasia, the severity of the epithelial changes or the presence of carcinoma will be correlated with the clinical appearance. For example, a thin white plaque is less likely to harbour high-grade dysplasia than a thick white plaque or erythematous, ulcerated and indurated regions. Multiple biopsy samples may be required if the lesion is extensive or shows a variety of clinical presentations1,2,5 (Fig. 3). Classic teaching includes selecting a site at the periphery of the lesion to ensure inclusion of healthy tissue in the sample. However, the principle guiding site selection should be acquisition of the most representative sample; an attempt to include tissue from the periphery may inadvertently lead to underdiagnosis.1,5
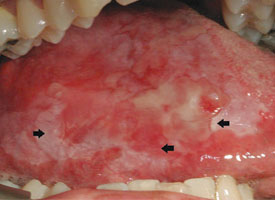 Figure 3: Asymptomatic ulcerated erythroleukoplakia diffusely affecting the right lateral tongue. Representative incisional biopsies were obtained and showed, from left to right, areas of thick keratosis, erythema and induration.
Figure 3: Asymptomatic ulcerated erythroleukoplakia diffusely affecting the right lateral tongue. Representative incisional biopsies were obtained and showed, from left to right, areas of thick keratosis, erythema and induration.
The technique used for incisional biopsy is usually straightforward and is illustrated step-by-step (Fig. 4a to 4g). An elliptical incision, with a length-to-width ratio of 3:1, is made with a size 15 scalpel blade. The elliptical shape facilitates primary-intention closure. The inferior incision is made first, so that hemorrhage does not obscure the surgical field. The anterior tip of the ellipse is gently lifted with tissue forceps, and the base is severed.
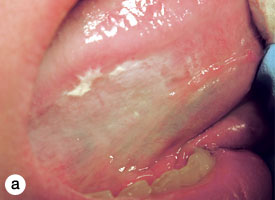
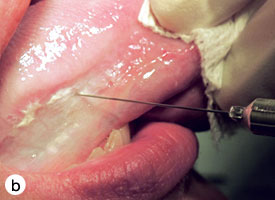
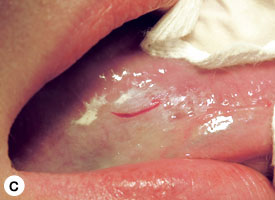
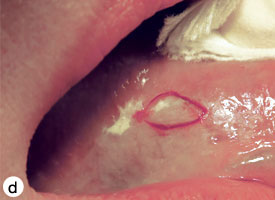
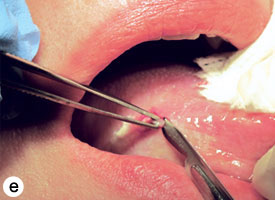
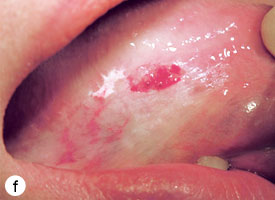
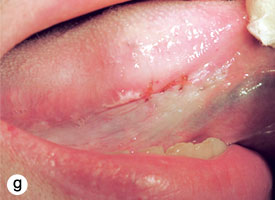
Figure 4. Homogenous leukoplakia affecting the right lateral and ventral areas of the tongue (a). Incisional biopsy is used to sample a thick, keratotic region at the anterior aspect of the tongue. Infiltration of local anesthetic (b) is followed by tracing of an ellipse (c and d). The anterior edge of the ellipse is gently raised with tissue forceps, which allows detachment of a canoe-shaped sample (e and f). Hemostasis is achieved with single interrupted sutures (g).
Excisional Biopsy
Excisional biopsy is the complete removal of a lesion for functional and aesthetic purposes, as well as to confirm the clinical diagnosis. This is appropriate only if the lesion is almost certainly benign.6 The size, accessibility and regional anatomy of the lesion must all be considered. Small, pedunculated, exophytic lesions in accessible areas are excellent candidates for excisional biopsy. An ellipse is traced around the lesion, with the blade angled toward the centre of the lesion (Fig. 5). This produces a wedge-shaped specimen that is deepest under the centre of the lesion and leaves a wound that is simple to close.
Punch Biopsy
Punch biopsy may be used for either incisional biopsy or excision of a small lesion at an accessible site. The lateral tongue and buccal mucosa are appropriate sites for punch biopsy, as it must be feasible for the device to approach the mucosal surface perpendicularly. The punch is placed on the lesional tissue, and a downward, twisting motion is applied (Fig. 6). The tissue core is then severed at the base with curved scissors. The circular wound makes approximating the edges more difficult than is the case with an elliptical shape. Punch biopsy is not appropriate for vesiculobullous diseases, as the twisting action would detach the epithelium and prevent proper assessment of the interface between epithelium and connective tissue that is necessary for subclassification of such lesions.
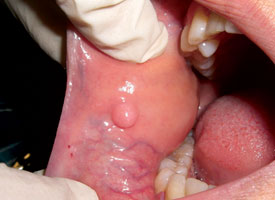 Figure 5. Pink nodule affecting the buccal mucosa and located adjacent to the occlusal plane is consistent with an irritation fibroma. Elliptical excisional biopsy facilitates primary-intention healing.
Figure 5. Pink nodule affecting the buccal mucosa and located adjacent to the occlusal plane is consistent with an irritation fibroma. Elliptical excisional biopsy facilitates primary-intention healing.
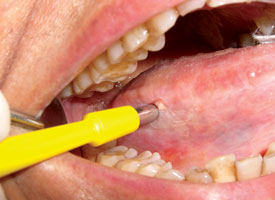 Figure 6. Thin, homogenous leukoplakia affecting the lateral and ventral areas of the tongue is suitable for incisional punch biopsy. The punch biopsy device is applied with a downward and twisting motion.
Figure 6. Thin, homogenous leukoplakia affecting the lateral and ventral areas of the tongue is suitable for incisional punch biopsy. The punch biopsy device is applied with a downward and twisting motion.
Electrosurgery and Laser Techniques
Electrosurgery and laser techniques produce thermal artifacts that may hamper histologic interpretation; accordingly, these methods should be used with caution for diagnostic biopsy or when information from the margins is required. Lasers may be of great value, however, in managing a wound left by scalpel biopsy in areas of the mouth where closure is difficult or inappropriate. A laser produces a zone of thermal coagulation smaller than that of electrosurgery, but still, a 0.5-mm margin should be maintained between the cut and the representative area to be sampled. This technique may produce good local hemostasis and minimal postoperative discomfort (Fig. 7).
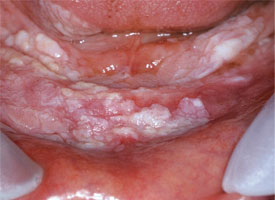 Figure 7: Widespread, thick leukoplakia affecting the floor of the mouth and the mandibular gingiva is excised with a laser to help minimize hemorrhage and discomfort (photograph courtesy of Dr. S. Trembley).
Figure 7: Widespread, thick leukoplakia affecting the floor of the mouth and the mandibular gingiva is excised with a laser to help minimize hemorrhage and discomfort (photograph courtesy of Dr. S. Trembley).
Adjunctive Techniques
Brush biopsy has been advocated as a screening modality for innocuous lesions that may otherwise not be sampled. With this method, a stiff brush is used to collect cells from all epithelial layers through application of firm pressure with a rotational movement. Pinpoint bleeding indicates sufficient depth of cell collection.3,4 The sample is transferred to a glass slide and sent to the laboratory for analysis.3,4,7 If atypical cells are found, conventional biopsy is also required. Routine use of brush biopsy remains debatable, especially given the accessibility of oral lesions for conventional biopsy.2
Adjunctive diagnostic aids, including nuclear stains and light sources, are marketed to assist in the detection of early cancerous changes. Staining with a metachromatic dye (toluidine blue) can help to define subtle erythroplakia, which facilitates complete excision. Pilot studies with autofluorescence, to delineate field changes around high-grade dysplasia and cancers and thereby guide excision, have been promising, but the potential for effects on disease recurrence requires further study.8
Special Considerations and Pitfalls
Rapid proliferation of a carcinoma can outpace the nutrient supply, resulting in necrosis and ulceration. Biopsy of a sample from the centre of such an ulcer, or its base, results in a nonspecific diagnosis. Rather, the adjacent intact mucosa, often configured as a raised border, should be the biopsy target. Similarly, for vesiculobullous disorders, there may be widespread ulceration, but diagnosis necessitates sampling intact mucosa in proximity to the ulceration. Extensive epithelial sloughing can make this type of biopsy extremely challenging.
The bound tissues of the gingiva and hard palate preclude the use of sutures in these areas. Healing is by secondary intention, and denuded bone and associated discomfort may persist for several weeks. Palatal biopsy must take into account the underlying vascular anatomy. Gingival biopsy may result in recession and ultimately esthetic defects and exposure of the root.
Biopsy of the lip may be accompanied by brisk hemorrhage, and the mobility of the labial tissues further complicates what would otherwise be a simple procedure. Local hemostasis and tissue stabilization are managed better if the assistant firmly grips the lip by placing the thumb and index finger on either side of the lesion. Although biopsy is unlikely to produce a major deformity, esthetics may be compromised, especially if an incision crosses the vermilion border. Local paresthesia may occur if nerves are severed.
Tongue lesions present similar difficulties. An assistant can stabilize the tongue by wrapping the tip with gauze. For closure of tongue wounds, deep sutures should be placed when indicated, and mucosal sutures should be placed fairly close to each other. Inadequate suturing will quickly lead to reopening of the wound, with resultant hemorrhage and delayed healing.
Biopsy Procedure
Armamentarium
The minimal requirements are as follows (Fig. 8):
- blade handle and no. 15 blade
- fine tissue forceps (preferably Adson forceps)
- syringe and local anesthetic
- retractor appropriate for the site
- sutures, if needed
- needle driver
- curved scissors
- hemostatic agents (silver nitrate or absorbable gelatin sponge)
- gauze sponges
- specimen bottle containing 10% neutral buffered formalin
- biopsy data sheet
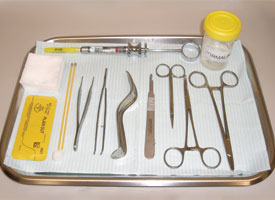 Figure 8. Basic armamentarium for biopsy.
Figure 8. Basic armamentarium for biopsy.
Consent
Verbal and written informed consent should be obtained before any biopsy. The surgical details should be discussed with the patient, as well as potential complications (usually discomfort, swelling, infection and hemorrhage). Surgery adjacent to nerves can result in transient or permanent paresthesia. Esthetic considerations are applicable to the gingival esthetic zone and the vermilion border of the lip. Procedures in the floor of the mouth can damage or obstruct the salivary ducts. Reactive lesions may recur, and re-excision may be required.
Anesthesia
Administration of local anesthetic is generally simple and straightforward. Lidocaine 2% combined with epinephrine (for local hemostasis), at a ratio of 1:100 000, is commonly used. Typically, less than one carpule, or 1.8 mL, is required. The local anesthetic should be infiltrated deep to or in a field around the biopsy site. Intralesional administration of anesthetic should be avoided, as it may distort the tissue or produce artifact. Regional block may also be used, although the benefit of epinephrine is lost.
Handling of the Specimen
The specimen must be gently grasped with forceps or secured with a traction suture.1 Toothed forceps are acceptable if care is taken and the area grasped is away from the primary site of interest (see Fig. 4e). Suction devices, if required, should be used with care to prevent loss of the specimen and suction-induced artifact. Gauze mounted on a hemostat is sufficient in most cases.
Tissue artifacts may originate from a number of sources, but all interfere with histologic interpretation. Crush artifact is common and is typically due to inappropriate compression from forceps. Often the forceps teeth will leave an impression in the tissue (Fig. 9a). Thermal artifact accompanying use of laser or electrosurgery devices obscures cytologic details around the perimeter of the specimen (Fig. 9b) and is especially problematic when information about the status of the margin is required. In colder climates, tissues may freeze during mailing; freezing severely distorts the histologic architecture (Fig. 9c).
 Figure 9a: Crush artifact, whereby excessive compressive force has produced an impression of the teeth of the tissue forceps within the tissue.
Figure 9a: Crush artifact, whereby excessive compressive force has produced an impression of the teeth of the tissue forceps within the tissue.
 Figure 9b: Cautery artifact caused by a laser device distorts the cytologic detail around the perimeter of the specimen.
Figure 9b: Cautery artifact caused by a laser device distorts the cytologic detail around the perimeter of the specimen.
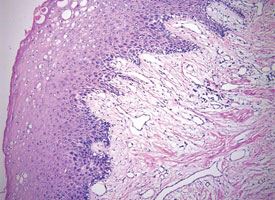 Figure 9c: Formation of ice crystals distorts the tissue architecture in a sample that became frozen while being shipped to the pathologist.
Figure 9c: Formation of ice crystals distorts the tissue architecture in a sample that became frozen while being shipped to the pathologist.
On occasion, it may be necessary to specify the orientation of the specimen if information about the completeness of excision is required, for example, when excising high-grade epithelial dysplasia. Orientation is accomplished by placing one or several sutures on known margins. If the specimen is thin, it is advisable to place it on a piece of paper, with the connective tissue side down, for at least 1 minute to ensure that the sample stays flat during fixation. When more than one specimen is collected, the specimens should be clearly distinguished either by placing identifying sutures in each specimen or by submitting separate specimen containers.1
The specimen should be placed in 10% neutral buffered formalin with at least 20 times the volume of the sample to avoid improper fixation or autolysis. Water, saline, alcohol, surface disinfectant, local anesthetic solution and mouth rinse cannot fix tissue properly and should not be used for this purpose. The fixative should not be changed or diluted.1
For definitive diagnosis of vesiculobullous disorders, tissue is obtained for conventional biopsy examination as well as direct immunofluorescence studies. The latter require tissue that has been stored in Michel’s solution. The sample must be received by the lab within 48 hours as the solution is water-based and does not preserve the tissue.
Hemostasis
Small biopsy wounds may be closed with a few single interrupted sutures using resorbable plain gut. Scaffolding agents, such as absorbable gelatin sponge, are useful for gingival and palatal biopsies, where the bound mucosa precludes placement of sutures. A denture or preconstructed acrylic base or stent can be helpful to protect palatal surgical sites. Small wounds in the floor of the mouth heal well without primary closure, and hemostasis may be achieved with a chemical cautery agent such as silver nitrate. Hemostasis may also be achieved with a laser or electrosurgery unit.
Postoperative Instructions
Standard postoperative instructions should be provided to the patient, including generic guidelines for eating, brushing, pain control and bleeding (Appendix 1). Emergency contact information should be made available.
Submission of Biopsy
The specimen should always be accompanied by pertinent clinical information, including the patient’s demographic data, clinical appearance, location of the lesion and any relevant medical history. Including a colour photograph of the lesion can be helpful.1
Materials should be sent by courier to minimize delay in diagnosis and to prevent freezing artifact, which may occur if the specimen is left in a mailbox or transported without temperature regulation. If the specimen is to be sent through the post, precise details of the regulations governing pathological specimens, available from the post office, should be followed. Most regulations require a primary container that is tightly sealed and wrapped in sufficient absorbent material, such as paper towel, in case of leakage. The wrapped container should then be placed in a sealed plastic bag, which is then placed in a rigid outer container that can be secured by adhesive tape. The package should indicate “Human Tissue” or “Pathological Specimen” and “Fragile” or “Handle With Care,” with the complete name and address of the sender.
Follow-up and Reporting of Biopsy Result to the Patient
Patients should be seen 1 to 2 weeks postoperatively to ensure healing and to discuss the results of the biopsy. It is the responsibility of the clinician (not the assistant or secretary) to explain the diagnosis and any further management if necessary. If the microscopic diagnosis is inconsistent with the clinical impression, the clinician is strongly advised to discuss any concerns directly with the pathologist.
Conclusion
Tissue biopsy is an indispensable tool, as proper management of oral mucosal disease begins with diagnosis. Although a wide variety of biopsy techniques and devices exist, the ultimate underlying goal is to obtain a representative tissue sample to facilitate histologic interpretation. When in doubt, the patient should be referred to a specialist with expertise in the diagnosis and management of oral diseases, such as an oral pathologist or oral surgeon.
THE AUTHORS
References
- Melrose RJ, Handlers JP, Kerpel S, Summerlin DJ, Tomich CJ, American Academy of Oral and Maxillifacial Pathology. The use of biopsy in dental practice. The position of the American Academy of Oral and Maxillofacial Pathology. Gen Dent. 2007;55(5):457-61.
- Mashberg A, Samit A. Early diagnosis of asymptomatic oral and oropharyngeal squamous cancers.CA Cancer J Clin. 1995;45(6):328-51.
- Driemel O, Kunkel M, Hullmann M,von Eggeling F, Müller-Richter U, Kosmehl H, et al.Diagnosis of oral squamous cell carcinoma and its precursor lesions. J Dtsch Dermatol Ges. 2007;5(12):1095-100.
- Collaborators from Royal College of Dental Surgeons of Ontario. Evidence-based clinical recommendations regarding screening for oral squamous cell carcinomas. Supplement to Aug/Sept 2010 isssue of Dispatch magazine.
- Poh CF, Samson Ng, Berean KW,Williams PM, Rosin MP, Zhang L.Biopsy and histopathologic diagnosis of oral premalignant and malignant lesions. J Can Dent Assoc. 2008;74(3):283-8.
- Oliver RJ, Sloan P, Pemberton MN. Oral biopsies: methods and applications. Brit Dent J. 2004;196(6):329-33.
- Patton LL. The effectiveness of community-based visual screening and utility of adjunctive diagnostic aids in the early detection of oral cancer. Oral Oncol. 2003;39(7):708-23.
- Poh CF, Zhang L, Anderson DW, Durham JS, Williams PM, Priddy RW, et al. Fluorescence visualization detection of field alterations in tumor margins of oral cancer patients. Clin Cancer Res. 2006;12(22):6716-22.
