Abstract
Patients undergoing cancer chemotherapy are living longer and with better quality of life, and they require dental care both during and after their treatments. Bisphosphonates have been associated with drug-related osteonecrosis of the jaw (ONJ) since the discoveries of Marx in 2003 and Ruggiero and Woo in 2008. Recent literature has indicated a similar association with nonbisphosphonate drugs used in cancer therapy. Denosumab, an osteoclast inhibitor with applications in orthopedics and oncology, causes ONJ at a rate comparable to that for intravenously administered bisphosphonates. Case reports and drug agency records have indicated a correlation between ONJ and the neoangiogenesis inhibitors bevacizumab and sunitinib, which are used to treat many common cancers. The pharmacologic mechanisms of these 3 drugs appear distinct, yet a common effect on bone metabolism may occur in susceptible hosts. This review explores the mechanisms of these drugs that could lead to ONJ, according to current scientific understanding. The American Academy of Oral and Maxillofacial Surgeons has provided detailed recommendations for the management of bisphosphonate-related ONJ, which we suggest should also be applied in the management of patients with exposure to denosumab, bevacizumab and sunitinib.
The management of drug-related osteonecrosis of the jaw (ONJ) has become a challenge for many dentists in recent years.1,2 Painful, infected necrotic bone lesions that exhibit prolonged healing dominate the clinical picture.3 Since the discoveries of Marx4 in 2003 and Ruggiero and Woo5 in 2008, bisphosphonates have become well known as playing a critical role in the pathogenesis of drug-related ONJ,6 a condition that creates a severe burden on patients' quality of life.7 Bisphosphonates are effective drugs that are widely used in orthopedics and oncology for the treatment of osteoporosis, Paget disease of bone and metastatic bone lesions.1,7-9 Reid and Cornish1 reported drug-related ONJ in about 5% of patients whose treatment for metastatic malignancies involved the use of bisphosphonates. Dental surgical procedures, longer duration of bisphosphonate therapy and poor oral hygiene have all been mentioned as risk factors for ONJ.1,3,10
Researchers have recently discovered additional drugs that may be associated with a risk for ONJ,11-25 including the monoclonal antibodies denosumab and bevacizumab and the multikinase inhibitor sunitinib. According to Stopeck and colleagues,13 Henry and colleagues26 and Fizazi and colleagues,27 the incidence of ONJ following denosumab therapy is similar to that following bisphosphonate therapy. The reports of bevacizumab- and sunitinib-associated ONJ are still anecdotal.16-18,20,21,23-25,28,29 According to recent drug information,30 precautions should be taken when performing dentoalveolar surgery or periodontal treatment on patients receiving these drugs, but no recommendations are currently available for the management of patients exposed to these drugs. In addition, the exact mechanisms of the pathogenesis of drug-related ONJ remain unclear.1,3,31
This review presents the most recent scientific information regarding the pharmacokinetics of the drugs associated with ONJ, as well as the current (though limited) understanding of the etiology of drug-related ONJ, and explains how drugs with different targets may cause the same clinical presentation of ONJ.
Bone Homeostasis and Mechanisms of Drug Interactions with Bone Turnover
Because ONJ is a lesion of the bone, it is important to review some basic information about the physiologic process of bone homeostasis. This dynamic, lifelong process is required for bone health, function and, when necessary, repair. Osteoclasts and osteoblasts are the cells responsible for bone resorption and apposition. Osteoblasts differentiate from osteocytes, whereas osteoclasts originate from the monocyte-macrophage lineage under the influence of cytokine growth factors, especially macrophage colony-stimulating factor (M-CSF), receptor activator of nuclear factor κ-B ligand (RANKL) and vascular endothelial growth factor (VEGF) (Fig. 1).12,15 The activity, differentiation and survival of osteoclasts are primarily dependent on exposure to RANKL,1,12,22,32 and these cells are inhibited by either the absence of RANKL or the presence of osteoprotegerin, which acts as a decoy receptor for RANKL.12,15,32,33 In turn, osteoblast activity is increased by mediators generated under osteoclast activity.15 These mechanisms are under the systemic control of the parathyroid hormone and vitamin D, which must be metabolized to its active form, calcitriol, by key enzymes that are expressed in the liver, the kidneys and many other cells (e.g., macrophages).34-36 Among various other mediators, M-CSF, VEGF, RANKL and activated vitamin D also play a role in the activation and chemotaxis of immunocompetent cells derived from the monocyte-macrophage lineage (Fig. 1).32,36-38
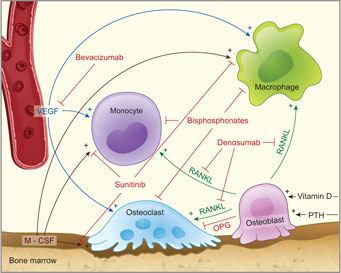 Figure 1: Physiology of bone homeostasis and mechanisms of drug interactions with bone turnover. M-CSF = macrophage colony-stimulating factor, OPG = osteoprotegerin, PTH = parathyroid hormone, RANKL = receptor activator of nuclear factor κ-B ligand.
Figure 1: Physiology of bone homeostasis and mechanisms of drug interactions with bone turnover. M-CSF = macrophage colony-stimulating factor, OPG = osteoprotegerin, PTH = parathyroid hormone, RANKL = receptor activator of nuclear factor κ-B ligand.
Because of their chemical structure, bisphosphonates can adhere firmly to the bone matrix,12 which explains their initially rapid removal from plasma and their long half-life in the bone.15,39,40 However, osteoclastic activity leads to the release of bisphosphonates from the bone matrix and locally high concentrations. Nitrogen-containing bisphosphonates (e.g., zoledronate) and nonnitrogen- containing bisphosphonates (e.g., clodronate) must be considered separately,8 because they differ in structure, pharmacodynamics and preferred therapeutic use.3,8 Non-nitrogencontaining bisphosphonates are primarily used to treat orthopedic disorders such as osteoporosis and Paget disease,3,10 whereas nitrogen-containing bisphosphonates are a component of supportive cancer therapy, facilitating the management of cancer-related morbidities such as hypercalcemia, pathological fractures and bone pain.3,7 The pharmacologic effect of non-nitrogencontaining bisphosphonates induces cell death, whereas nitrogen-containing bisphosphonates inhibit key enzymes of the cholesterol synthesis pathway.8,15,39,41 Cholesterol precursors in turn are needed for the proper functioning of cell signalling pathways.12,22,42
Among the nonbisphosphonates, denosumab acts on RANKL to inhibit the formation and activity of osteoclasts (Fig. 1) and is used to treat advanced osteoporosis and bone metastases. 12,13,22,26,27 Bevacizumab targets VEGF and is intended to prevent blood vessel growth.20 It is used in the treatment of selected advanced colon, lung, renal and central nervous system tumours and plays a developing role in the management of breast and ovarian cancers.19,24,29,31 Bevacizumab is also injected intraocularly for treatment of macular degeneration.24 Sunitinib, which belongs to the group of tyrosine kinase inhibitors,16,21 inhibits neoangiogenesis by interfering with the VEGF receptor, the M-CSF receptor and other pathways (Fig. 1).16 It has been approved for the treatment of renal cell cancer, some neuroendocrine tumours and gastrointestinal stromal tumours.21-23,30
Etiology of ONJ and possible overlapping effects of bisphosphonates, denosumab, bevacizumab and sunitinib
All 3 of these drugs have been reported to cause drug-related ONJ when administered in isolation (Table 1) or to increase the severity of ONJ when given in conjunction with bisphosphonates. 13,16-21,23-29 The British and French drug regulatory agencies have recognized 55 bevacizumabassociated cases of ONJ among approximately 800,000 patients undergoing bevacizumab treatment.30 Sunitinib-related ONJ was found in 27 of approximately 100,000 patients undergoing sunitinibtreatment.30
Table 1 Studies and case reports presenting drug-induced osteonecrosis of the jaw without concomitant administration of bisphosphonates
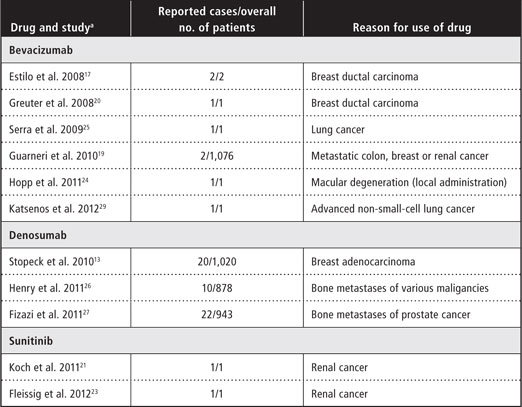
aWithin the section for each drug, references are ordered chronologically by date of publication.
The American Academy of Oral and Maxillofacial Surgeons (AAOMS) defines bisphosphonate-related ONJ on the basis of 3 criteria: current or prior exposure of the patient to bisphosphonates, presence of a necrotic bone lesion for at least 8 weeks and no history of irradiation of the involved bone.3,9 In addition, as described above, there have been reports of drug-related ONJ without exposure to bisphosphonates in patients under active or previous treatment with denosumab, bevacizumab or sunitinib (Table 1). At present, there is insufficient evidence in the literature to draw reliable conclusions about a potential cause-and-effect relationship between bevacizumab or sunitinib and ONJ, especially given that ONJ has also been reported to evolve without exposure to any of these drugs (although this has occurred only rarely).1 However, the literature does suggest that concurrent treatment with a bisphosphonate combined with either bevacizumab or sunitinib may increase the odds for development of ONJ and may also shorten the latency period.10,16,18,28,43,44 The clinical manifestations of denosumab-related ONJ and the relative risk of this condition are similar to those reported for the bisphosphonate zoledronic acid.13,26,27
Many authors have tried to explain the etiology of drug-related ONJ. In this regard, it is noteworthy that osteoclast function is naturally inhibited in osteopetrosis, a condition in which involvement of ONJ has not been described.12,15 Therefore, impaired osteoclastic activity cannot be the only cause of ONJ.
Although bisphosphonates, bevacizumab and sunitinib all have antiangiogenic effects,8,21,23 ONJ lesions exhibit intact vasculature on histologic examination.22 Furthermore, denosumab is not known to inhibit vascular formation and is nonetheless significantly associated with occurrence of ONJ.15 As such, impaired vascularization may play only a minor role in development of ONJ.
The mucosal toxicity of bisphosphonates is indicated by the common occurrence of esophagitis as an adverse effect of exposure to these drugs.31 In the oral cavity, epithelial cells are exposed to bisphosphonates not only systemically (via the plasmabound pharmacon) but also through steady release of the drug from the adjacent bone.12,31,39 This exposure may disrupt cell function and promote epithelial breakdown. Brunello and colleagues,16 Bozas and colleagues18 and Hoefert and Eufinger28 reported the rapid development of ONJ in patients who were concurrently receiving bisphosphonates and sunitinib, and they highlighted the strong potential of sunitinib to cause mucositis. This indicates that destruction of the epithelial barrier may be an important step in the development of drugrelated ONJ.
Bacterial invasion by Actinomyces may be a decisive factor in the etiology of higher-grade drug-related ONJ.45 Bacterial colonization with Actinomyces is virtually omnipresent in ONJ tissue samples.1,12 Actinomyces has also been found in sunitinib- and bevacizumab-related ONJ.17,21 Although Actinomyces is a regular colonizer of the oral cavity,45 severe infections, such as actinomycosis, are rare.45,46 Hansen and colleagues45 and Smith and colleagues47 suspected that accumulation of Actinomyces in the setting of ONJ may represent an opportunistic infection, although these bacteria have previously been associated with bone infection.45,46 Kos and colleagues48 compared osteomyelitis lesions in 18 patients who had undergone bisphosphonate treatment for metastatic cancer and 11 patients who had not undergone such treatment and found significantly greater colonization of necrotic bone with Actinomyces in the bisphosphonate group. This finding raises the question of whether local malfunction of the immune system in the defence bacteria may promote the development of drug-related ONJ.
Macrophages are the first line of defence against invading microorganisms. They activate the adaptive immune system and are capable of triggering the immune response by inducing chemotaxis of various immunocompetent cells.49 The biochemical and physiological features of osteoclasts and macrophages are similar, and there is evidence that they react alike to drug exposure.12,32,50,51 As stated above, M-CSF and VEGF can induce monocyte and macrophage differentiation and chemotaxis. 32,51 An important step in their activation is the recognition of exotoxins and endotoxins, such as bacterial lipopolysaccharide, by Toll-like receptors. 12,22,42,52 Activation of these receptors induces and enhances the host's defence mechanisms.53 This pathway can be further enhanced by the additional stimulus of calcitriol (activated vitamin D).53
The host immune response, which involves the cells and mechanisms described above, usually prevents bacterial invasion following epithelial damage. However, in certain ways, all of the drugs discussed in this review can interfere with the local innate immune system (Fig. 1).
Monocytes and macrophages have been shown to be impaired by exposure to bisphosphonates,12 which may lead to ineffective host defence against invading microorganisms.12 Furthermore, the peripheral activation of vitamin D may be diminished when the number of functioning macrophages is reduced, because, as stated above, monocytes and macrophages produce enzymes that can convert vitamin D into calcitriol.35 The decrease in peripherally available calcitriol may cause this mechanism of stimulating the immune system to fail, further inhibiting the ability of macrophages to effectively prevent bacterial invasion.12 However, to the best of the authors' knowledge, no studies have shown that decreased vitamin D and calcitriol levels in vivo are associated with ONJ in isolation. In a recent study, Balla and colleagues54 found a weak correlation between serum levels of vitamin D and the location of ONJ in the maxilla or mandible among patients treated with bisphosphonates. Other implications of these findings remain unclear.
Macrophage chemotaxis and osteoclast differentiation may subside after exposure to bevacizumab, which inactivates VEGF (Fig. 1).38,44 The proper functioning of important receptors of the innate immune system, such as Tolllike receptors, and of receptors that control the differentiation of osteoclasts and the monocytemacrophage lineage, such as the M-CSF and VEGF receptors, may be inhibited by the use of sunitinib (Fig. 1).12,21,55 Finally, the inactivation of RANKL by denosumab decreases osteoclast activity, as well as macrophage mobility and chemotaxis (Fig. 1).12,15 All of these mechanisms may work together, predisposing the bone to development of ONJ through a decrease in bone turnover and inhibition of host defence mechanisms.7,33,41
Discussion
Recent findings may imply that disturbance of bone homeostasis and immunologic factors play a role in the pathogenesis of drug-related ONJ. Although the numbers of ONJ cases associated with denosumab, bevacizumab and sunitinib treatment are still low, the number of published reports is growing, which suggests that treatment with these drugs may increase the odds for occurrence of ONJ. As advanced cancer therapy enables patients to live longer and better, they will require ongoing dental care. Dentists and oral surgeons can expect to encounter an increasing number of patients who are receiving treatments potentially toxic to bone but who also require good dental care. For any patient with a history of metastatic colon, breast, lung, ovarian or renal cancer, it is advisable for the general dentist to consult with the treating oncologist before performing any procedures, for better risk assessment.19,24,29 In the management of patients exposed to bisphosphonates, as well as to denosumab, bevacizumab or sunitinib, it is strongly recommended that surgical dental procedures and periodontal treatment be executed with special care and adherence to the AAOMS guidelines for the management of bisphosphonate-associated osteonecrosis of the jaw.3 The dental profession has been instrumental in describing and researching the implications of ONJ,6 and knowledge about further developments, including the etiology of ONJ, will enable dentists to better manage and prevent this condition, thereby increasing the quality of life of cancer patients and cancer survivors.
THE AUTHORS
References
- Reid IR, Cornish J. Epidemiology and pathogenesis of osteonecrosis of the jaw. Nat Rev Rheumatol. 2012;8(2):90-6.
- Siddiqi A, Payne AG, Zafar S. Bisphosphonate-induced osteonecrosis of the jaw: a medical enigma? Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108(3):e1-8. Epub 2009 Jul 1.
- Ruggiero SL, Dodson TB, Assael LA, Landesberg R, Marx RE, Mehrotra B. American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws--2009 update. J Oral Maxillofac Surg. 2009;67(5 Suppl):2-12.
- Marx RE. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg. 2003;61(9):1115-7.
- Ruggiero SL, Woo SB. Biophosphonate-related osteonecrosis of the jaws. Dent Clin North Am. 2008;52(1):111-28, ix.
- Ruggiero SL, Fantasia J, Carlson E. Bisphosphonate-related osteonecrosis of the jaw: background and guidelines for diagnosis, staging and management. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102(4):433-41. Epub 2006 Jul 31.
- Vahtsevanos K, Kyrgidis A, Verrou E, et al. Longitudinal cohort study of risk factors in cancer patients of bisphosphonate-related osteonecrosis of the jaw. J Clin Oncol. 2009;27(32):5356-62.
- Roelofs AJ, Thompson K, Gordon S, Rogers MJ. Molecular mechanisms of action of bisphosphonates: current status. Clin Cancer Res. 2006;12(20 Pt 2):6222s-6230s.
- Colella G, Campisi G, Fusco V. American Association of Oral and Maxillofacial Surgeons position paper: Bisphosphonate-Related Osteonecrosis of the Jaws-2009 update: the need to refine the BRONJ definition. J Oral Maxillofac Surg. 2009;67(12):2698-9.
- Bonacina R, Mariani U, Villa F, Villa A. Preventive strategies and clinical implications for bisphosphonate-related osteonecrosis of the jaw: a review of 282 patients. J Can Dent Assoc. 2011;77:b147.
- Chtioui H, Lamine F, Daghfous R. Teriparatide therapy for osteonecrosis of the jaw. N Engl J Med. 2011;364(11):1081-2; author reply 1082.
- Pazianas M. Osteonecrosis of the jaw and the role of macrophages. J Natl Cancer Inst. 2011;103(3):232-40. Epub 2010 Dec 28.
- Stopeck AT, Lipton A, Body JJ, Steger GG, Tonkin K, de Boer RH, et al. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J Clin Oncol. 2010;28(35):5132-9. Epub 2010 Nov 8.
- Fusco V, Galassi C, Berruti A, Ciuffreda L, Orgeta C, Ciccone G, et al. Osteonecrosis of the jaw after zoledronic acid and denosumab treatment. J Clin Oncol. 2011;29(17):e521-2; author reply e523-4. Epub 2011 May 2.
- Baron R, Ferrari S, Russell RG. Denosumab and bisphosphonates: different mechanisms of action and effects. Bone. 2011;48(4):677-92. Epub 2010 Dec 9.
- Brunello A, Saia G, Bedogni A, Scaglione D, Basso U. Worsening of osteonecrosis of the jaw during treatment with sunitinib in a patient with metastatic renal cell carcinoma. Bone. 2009;44(1):173-5. Epub 2008 Sep 24.
- Estilo CL, Fornier M, Farooki A, Carlson D, Bohle G, 3rd, Huryn JM. Osteonecrosis of the jaw related to bevacizumab. J Clin Oncol. 2008;26(24):4037-8.
- Bozas G, Roy A, Ramasamy V, Maraveyas A. Osteonecrosis of the jaw after a single bisphosphonate infusion in a patient with metastatic renal cancer treated with sunitinib. Onkologie. 2010;33(6):321-3. Epub 2010 May 11.
- Guarneri V, Miles D, Robert N, Dieras V, Glaspy J, Smith I, et al. Bevacizumab and osteonecrosis of the jaw: incidence and association with bisphosphonate therapy in three large prospective trials in advanced breast cancer. Breast Cancer Res Treat. 2010;122(1):181-8. Epub 2010 Apr 2.
- Greuter S, Schmid F, Ruhstaller T, Thuerlimann B. Bevacizumab-associated osteonecrosis of the jaw. Ann Oncol. 2008;19(12):2091-2. Epub 2008 Oct 31.
- Koch FP, Walter C, Hansen T, Jager E, Wagner W. Osteonecrosis of the jaw related to sunitinib. Oral Maxillofac Surg. 2011;15(1):63-6.
- Compston J. Pathophysiology of atypical femoral fractures and osteonecrosis of the jaw. Osteoporos Int. 2011;22(12):2951-61. Epub 2011 Oct 14.
- Fleissig Y, Regev E, Lehman H. Sunitinib related osteonecrosis of jaw: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2012 Jan 2. [Epub ahead of print]
- Hopp RN, Pucci J, Santos-Silva AR, Jorge J. Osteonecrosis after administration of intravitreous bevacizumab. J Oral Maxillofac Surg. 2012;70(3):632-5. Epub 2011 Jul 14.
- Serra E, Paolantonio M, Spoto G, Mastrangelo F, Tete S, Dolci M. Bevacizumab-related osteneocrosis of the jaw. Int J Immunopathol Pharmacol. 2009;22(4):1121-3.
- Henry DH, Costa L, Goldwasser F, Hirsh V, Hungria V, Scagliotti GV, et al. Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J Clin Oncol. 2011;29(9):1125-32. Epub 2011 Feb 22.
- Fizazi K, Carducci M, Smith M, Damiao R, Brown J, Karsh L, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet. 2011;377(9768):813-22. Epub 2011 Feb 25.
- Hoefert S, Eufinger H. Sunitinib may raise the risk of bisphosphonate-related osteonecrosis of the jaw: presentation of three cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;110(4):463-9. Epub 2010 Aug 9.
- Katsenos S, Christophylakis C, Psathakis K. Osteonecrosis of the jaw in a patient with advanced non-small-cell lung cancer receiving bevacizumab. Arch Bronconeumol. 2012;48(6):218-9. Epub 2012 Mar 17.
- Bevacizumab, sunitinib: osteonecrosis of the jaw. Prescrire Int. 2011;20(117):155.
- Reid IR, Bolland MJ, Grey AB. Is bisphosphonate-associated osteonecrosis of the jaw caused by soft tissue toxicity? Bone. 2007;41(3):318-20. Epub 2007 May 10.
- Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423(6937):337-42.
- Koch FP, Merkel C, Ziebart T, Smeets R, Walter C, Al-Nawas B. Influence of bisphosphonates on the osteoblast RANKL and OPG gene expression in vitro. Clin Oral Investig. 2012;16(1):79-86. Epub 2010 Oct 12.
- Doyle ME, Jan de Beur SM. The skeleton: endocrine regulator of phosphate homeostasis. Curr Osteoporos Rep. 2008;6(4):134-41.
- Hewison M, Burke F, Evans KN, Lammas DA, Samsom DM, Liu P, et al. Extra-renal 25-hydroxyvitamin D3-1alpha-hydroxylase in human health and disease. J Steroid Biochem Mol Biol. 2007;103(3-5):316-21.
- Hewison M, Zehnder D, Chakraverty R, Adams JS. Vitamin D and barrier function: a novel role for extra-renal 1 alpha-hydroxylase. Mol Cell Endocrinol. 2004;215(1-2):31-8.
- Mancini A, Koch A, Whetton AD, Tamura T. The M-CSF receptor substrate and interacting protein FMIP is governed in its subcellular localization by protein kinase C-mediated phosphorylation, and thereby potentiates M-CSF-mediated differentiation. Oncogene. 2004;23(39):6581-9.
- Aldridge SE, Lennard TW, Williams JR, Birch MA. Vascular endothelial growth factor receptors in osteoclast differentiation and function. Biochem Biophys Res Commun. 2005;335(3):793-8.
- Cornish J, Bava U, Callon KE, Bai J, Naot D, Reid IR. Bone-bound bisphosphonate inhibits growth of adjacent non-bone cells. Bone. 2011;49(4):710-6. Epub 2011 Jul 23.
- Lemound J, Eckardt A, Kokemuller H, von See C, Voss PJ, Tavassol F, et al. Bisphosphonate-associated osteonecrosis of the mandible: reliable soft tissue reconstruction using a local myofascial flap. Clin Oral Investig. 2011 Aug 5. [Epub ahead of print]
- Walter C, Al-Nawas B, Grotz KA, Thomas C, Thuroff JW, Zinser V, et al. Prevalence and risk factors of bisphosphonate-associated osteonecrosis of the jaw in prostate cancer patients with advanced disease treated with zoledronate. Eur Urol. 2008;54(5):1066-72. Epub 2008 Jun 26.
- Chappell WH, Steelman LS, Long JM, Kempf RC, Abrams SL, Franklin RA, et al. Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR inhibitors: rationale and importance to inhibiting these pathways in human health. Oncotarget. 2011;2(3):135-64.
- Aragon-Ching JB, Ning YM, Chen CC, Latham L, Guadagnini JP, Gulley JL, et al. Higher incidence of Osteonecrosis of the Jaw (ONJ) in patients with metastatic castration resistant prostate cancer treated with anti-angiogenic agents. Cancer Invest. 2009;27(2):221-6.
- Ayllon J, Launay-Vacher V, Medioni J, Cros C, Spano JP, Oudard S. Osteonecrosis of the jaw under bisphosphonate and antiangiogenic therapies: cumulative toxicity profile? Ann Oncol. 2009;20(3):600-1. Epub 2009 Feb 2.
- Hansen T, Kunkel M, Springer E, Walter C, Weber A, Siegel E, et al. Actinomycosis of the jaws--histopathological study of 45 patients shows significant involvement in bisphosphonate-associated osteonecrosis and infected osteoradionecrosis. Virchows Arch. 2007;451(6):1009-17. Epub 2007 Oct 20.
- Wong VK, Turmezei TD, Weston VC. Actinomycosis. BMJ. 2011;343:d6099.
- Smith MH, Harms PW, Newton DW, Lebar B, Edwards SP, Aronoff DM. Mandibular Actinomyces osteomyelitis complicating florid cemento-osseous dysplasia: case report. BMC Oral Health. 2011;11:21.
- Kos M, Brusco D, Kuebler J, Engelke W. Clinical comparison of patients with osteonecrosis of the jaws, with and without a history of bisphosphonates administration. Int J Oral Maxillofac Surg. 2010;39(11):1097-102.
- Zelkha SA, Freilich RW, Amar S. Periodontal innate immune mechanisms relevant to atherosclerosis and obesity. Periodontol 2000. 2010;54(1):207-21.
- Udagawa N. The mechanism of osteoclast differentiation from macrophages: possible roles of T lymphocytes in osteoclastogenesis. J Bone Miner Metab. 2003;21(6):337-43.
- Dore RK. The RANKL pathway and denosumab. Rheum Dis Clin North Am. 2011;37(3):433-52, vi-vii.
- Arbibe L, Mira JP, Teusch N, Kline L, Guha M, Mackman N, et al. Toll-like receptor 2-mediated NF-kappa B activation requires a Rac1-dependent pathway. Nat Immunol. 2000;1(6):533-40.
- Gambhir V, Kim J, Siddiqui S, Taylor M, Byford V, Petrof EO, et al. Influence of 1,25-dihydroxy vitamin D3 on TLR4-induced activation of antigen presenting cells is dependent on the order of receptor engagement. Immunobiology. 2011;216(9):988-96. Epub 2011 Apr 7.
- Balla B, Vaszilko M, Kosa J, Podani J, Takacs I, Tobias B, et al. New approach to analyze genetic and clinical data in bisphosphonate-induced osteonecrosis of the jaw. Oral Dis. 2012 Jan 31. doi: 10.1111/j.1601-0825.2012.01912.x. [Epub ahead of print]
- Mashkani B, Griffith R, Ashman LK. Colony stimulating factor-1 receptor as a target for small molecule inhibitors. Bioorg Med Chem. 2010;18(5):1789-97. Epub 2010 Jan 28.






