Abstract
With the increasing use of the bisphosphonate class of drugs, dental professionals are encountering more cases of bisphosphonate-related osteonecrosis of the jaw (BRONJ). The C-terminal cross-linking telopeptide (CTX) assay is a serologic test to predict the risk of BRONJ. This paper examines the effectiveness of CTX as a biochemical marker for BRONJ and its utility to the dentist in establishing appropriate treatment plans for patients with a history of bisphosphonate use. Alternative means of assessing the risk of BRONJ are discussed, and 2 case vignettes are presented (vignette 1, vignette 2) to demonstrate dental treatment planning for patients with a history of bisphosphonate use, in the context of specific CTX results.
A Look at Bisphosphonates
Since the introduction of the bisphosphonate class of drugs in 1969,1 the number of patients taking these agents has grown at a significant and rapidly accelerating rate. More specifically, since alendronate, risedronate and ibandronate were released into the market, over 225 million prescriptions for these 3 drugs have been written in the United States,2 with 27 million of these prescriptions dispensed in 2008 alone.1 With the increasing use of this drug class, researchers and clinicians are gaining a greater understanding of their indications for use, mechanisms of action, positive outcomes and adverse events. One critical adverse event associated with use of these drugs is bisphosphonate-related osteonecrosis of the jaw (BRONJ). This paper examines the serologic biochemical marker C-terminal cross-linking telopeptide (CTX) to determine its effectiveness as a predictor for the development of BRONJ and how CTX results influence dentists in the planning and execution of treatment.
Clinical Aspects of Bisphosphonate Therapy
Bisphosphonates are typically used to suppress bone turnover. Their primary action is to inhibit the resorption of bone through effects on osteoclasts.3,4 The reported pharmacodynamics involve suppression of activation and induction of apoptosis of these cells.5 Osteoclasts resorb bone containing dead or aged osteocytes and microfractures, a process that releases bone-forming chemical mediators such as cytokines, bone morphogenetic protein and insulin-like growth factors 1 and 2.6 Therefore, the inhibitory effects of bisphosphonates on osteoclasts prevent bone remodelling, repair and regeneration. The first generation of these drugs were the non–nitrogen containing bisphosphonates, including the oral and intravenous (IV) drugs etidronate and clodronate, which are now less favoured clinically because of their lower potencies relative to more recent drugs in this class.1 It is believed that the non-nitrogen containing bisphosphonates act by forming cytotoxic metabolites of adenosine triphosphate (ATP), thereby interrupting intracellular metabolic activity.4 In contrast, the nitrogen-containing bisphosphonates are more widely used clinically, both as oral and IV drugs. Their mechanism involves inhibition of the mevalonate pathway, which ultimately results in osteoclast dysfunction and apoptosis. Notably, the oral and IV bisphosphonates differ markedly in terms of their properties, half-lives and possible sequelae. Examples of IV bisphosphonates include pamidronate and zoledronate. The oral bisphosphonates include alendronate, risedronate and ibandronate.3,4,6
Clinical indications for the use of bisphosphonates include multiple myeloma, metastatic breast carcinoma,7 osteoporosis and osteopenia,5,8,9 Paget disease3 and bone metastasis secondary to prostate cancer, lung cancer and renal cell carcinoma.10 Pediatric indications include osteogenesis imperfecta and idiopathic juvenile osteoporosis and osteopenia associated with rheumatoid arthritis.4 Some authors have demonstrated potential antitumour effects by means of tumour cell apoptosis and inhibition of tumour invasion and adhesion.11 The lower-dose oral form has commonly been used for fracture prevention in patients with osteoporosis, whereas the more potent IV form is widely used for the treatment of multiple myeloma, Paget disease and metastatic carcinomas.3,9,10,12-14 Inherent with this class of drugs, an adverse event profile exists which is dependent on drug, route, dosage and frequency and duration of administration. Common adverse reactions include: upper gastrointestinal (e.g., abdominal pain, nausea, dyspepsia, constipation, diarrhea), musculoskeletal (e.g., bone, muscle, joint pain and/or cramping), and nervous (e.g., headache, dizziness). Reported warnings and precautions include: renal insufficiency, atypical fractures of the femur, atrial fibrillation, mineral deficiencies, and BRONJ.15-17 The latter is of great importance to the field of dentistry and is the focus of this article.
The frequency of BRONJ after dental extraction has been estimated at 0.09%–0.34% among patients taking oral bisphosphonates and 6.7%–9.1% among those taking IV formulations.3,12 Of the reported cases of BRONJ, an estimated 94% have been diagnosed in patients with a history of IV bisphosphonate therapy.18 The higher rate of adverse events with IV bisphosphonates may be due to the pharmacokinetics of these drugs at the target site. The oral form has a bioavailability of only 0.64%,6,8 and the occurrence of BRONJ is reportedly 500 times less likely with oral bisphosphonates than with high-dose IV forms.13 The long half-life of bisphosphonates (> 10 years), the rapid accumulation of IV bisphosphonates at their target sites and the bypassing of the first-pass effect of oral drugs may explain the greater risk with the IV form. Because of the high accumulation of bisphosphonates in bone with IV bisphosphonate administration, the osteoclast population is less likely to recover, and BRONJ may therefore be less amenable to treatment and recovery as compared to oral administration.6 Even among the oral formulations of these drugs, differences exist in the risk of BRONJ. Alendronate seems to have a greater affinity for bony tissues than other oral bisphosphonates, is more potent, and, most importantly, is more often prescribed and is therefore a more frequent BRONJ-causing agent.18 Among the IV bisphosphonates, zoledronate is most frequently associated with BRONJ.14 Marx and colleagues6 stated that oral alendronate is as potent as the IV drug pamidronate.
Despite the inherent risk of BRONJ with bisphosphonate therapy, it is important to emphasize the effectiveness of these drugs in specific clinical scenarios. For example, zoledronate has been shown to reduce the incidence of vertebral fractures, hip fractures and other fractures by 70%, 41% and 25%, respectively.3 Additionally, the number needed to treat to prevent one vertebral fracture was as low as 13. As such, the risk–benefit ratio greatly favours the use of these drugs in patients with osteoporosis.
Osteonecrosis of the Jaw
Osteonecrosis among patients taking bisphosphonates is most often observed in the jaw. Under normal conditions, the rate of bone turnover in the jaw is greater than in the skeleton as a whole.3 Consequently, reduced turnover secondary to intake of bisphosphonates considerably diminishes the regenerative and healing capacities of the jaws in response to injury, dental extraction or physiologic wear from occlusal forces. The Advisory Task Force on Bisphosphonate-Related Osteonecrosis of the Jaws of the American Association of Oral and Maxillofacial Surgeons, in its 2007 position paper, defined BRONJ as a condition in which (1) exposed, necrotic bone has been present in the maxillofacial region for longer than 8 weeks; (2) there is a history of current or past use of bisphosphonate; and (3) there is no history of radiation therapy to the jaws.1,4-6,8-10,13,14,19 The clinical presentation varies, and the patient may present with mobile dentition, soft-tissue inflammation, sinus tracts, neurosensory changes of the lip or purulent discharge; alternatively, the condition may be asymptomatic.5 Pain is correlated directly with infection, which is indicated by an inflammatory response.6 Frequently, species of Actinomyces,14 Moraxella and Eikenella have been found on debrided bone from BRONJ sites, which indicates that microbial infection may play a role in its etiology. Radiographic findings may include nonspecific changes, such as osteolysis, osteosclerosis, sclerotic lamina dura around the teeth, sclerotic bone over the mylohyoid ridge, mottled appearance of bone in the premolar and molar regions, persistent alveolar bone sockets or widening of the periodontal ligament.14 Precipitating factors for BRONJ include dental extractions (in almost 75% of cases), followed by periodontal disease, ill-fitting dentures and trauma to the tori.12-14 Advanced age, steroid therapy, compromised health and female sex have all been identified as risk factors; however, the last of these is likely related to the fact that 70% of bisphosphonate users are female.12 The risk for BRONJ follows a dose–response curve for bisphosphonate users, and is further increased with the frequency of administration. 14 Interestingly, 25% of cases of IV BRONJ are classified as spontaneous6, specifically not caused by traumatic injury, although many researchers hypothesize that an undetectable predisposing factor may in fact be the causative factor, such as untreated nonrestorable carious or abscessed dentition and/or the presence of widened periodontal ligaments.10 The differential diagnosis most commonly includes alveolar osteitis, sinusitis, gingivitis or periodontitis; periapical pathology; and lingual mandibular sequestration with ulceration.4,9
C-Terminal Cross-Linking Telopeptide
Table 1 Reference ranges for C-terminal cross-linking telopeptide (CTX) used by Quest Diagnostics (Madison, NJ)
| CTX reference range (pg/mL) | ||
| Age | Male | Female |
| Adult | ||
| 18–29 years | 87–1200 | 60–640 |
| 30–39 years | 70–780 | 60–650 |
| 40–49 years | 60–700 | 40–465 |
| Pediatric | ||
| 5–9 years | 574–1849 | 574–1849 |
| 10–13 years | 519–2415 | 519–2415 |
| 14–17 years | 435–2924 | 242–1291 |
The most widely used serologic biochemical marker for stratifying the risk of BRONJ among bisphosphonate users is the C-terminal cross-linking telopeptide (CTX) of type I collagen.1,2,5,6,10,12,18-20 In situations of increased bone turnover, type I collagen is degraded by osteoclasts, which releases CTX molecules. Collagen I is one of the most abundant constituents of bone, reaching up to 90%–98% of the organic matrix.5,6 It has been demonstrated that declines in serum levels of CTX can be quantified within weeks of initiation of bisphosphonate therapy.12 Although the potential of CTX studies to determine the risk of BRONJ is scientifically plausible, some authors have further correlated the predictive value of CTX with the number and size of potential lesions.6 Studies have shown that within 6 weeks of initiation of bisphosphonate administration at conventional dosages, CTX levels may decrease by 60%.12 In the articles retained for this paper (see Literature Review), CTX reference ranges vary widely, with some studies using a range of 50–370 pg/mL,1 and others, a range of 115 to 1,351 pg/mL.5 Quest Diagnostics (Madison, NJ) lists multiple reference ranges21 according to sex and age (see Table 1).
CTX values depend on age, sex, smoking status, ovulation, concurrent drug use, exercise, circadian rhythms,10 renal function3 and fasting states. For example, prednisone and methotrexate, a widely used combination therapy for certain autoimmune disorders (including multiple myeloma), further decreases osteoclast activity and suppresses bone turnover.6 Although most studies indicate that CTX is measured after fasting, to reduce variability of bone turnover markers,10 others have reported findings based on non-fasting CTX testing. It is believed that CTX levels vary little between fasting and non-fasting states compared with other serologic markers,18 but no data exist to allow determination of any statistically significant differences. In fact, some researchers have countered that the greatest variability in CTX is related to circadian rhythms and fasting states.18
Drug holidays of 3–6 months have been recommended for patients with a 3-year or greater history of bisphosphonate use.4,6,8,9,11 Patients taking bisphosphonates concurrently with steroid therapy are advised to take a 3-month drug holiday even if they have used the bisphosphonate for less than 3 years.11 CTX values increase by 25.9–26.4 pg/mL for each month of a drug holiday.10,20
Literature Review
The literature dealing with CTX as a predictor for BRONJ was searched using the PubMed (National Library of Medicine) and Cochrane Library databases, with the key words “ctx,” “bisphosphonate,” “osteonecrosis” and “bronj” (Table 2). Articles with the following characteristics were retained for analysis: publication date 2007 or later, human studies and publication in English.
Table 2 Results of the literature search
| Database searched | Key words used | No. of citations retrieved | No. of articles retained by search querya |
| PubMed (National Library of Medicine) | ctx AND bronj | 8 | 5 |
| bisphosphonate AND osteonecrosis AND ctx | 28 | 6 | |
| Web of Knowledge | Citation search: Marx AND and Cillo AND Ulloa AND 2007 | 1 | 1 |
| Cochrane Library | osteonecrosis AND bisphosphonate | 11 | 1 |
| Articles retrieved from references | NA | 4 | 4 |
NA = not applicable.
aArticles were retained for analysis if they had been published in 2007 or later, if they involved human studies and if the language of publication was English.
Standard of Care
Risk Tables
Table 3 Risk stratification table6
| CTX value (pg/mL) | Assigned risk |
| < 100 | High |
| 100–150 | Moderate |
| > 150 | Minimal |
Marx used CTX values to stratify patients into minimal-, moderate- and high-risk categories (see Table 3).6 This stratification of risk is repeatedly cited in literature on BRONJ and bisphosphonate use; in fact, over three-quarters of the articles retained from the literature search for this paper assigned risk according to the Marx table. A Web of Knowledge (Thomson Reuters; http://www.isiwebofknowledge.com) citation search on April 21, 2013, revealed that Marx’s 2007 article6 had been cited 219 times. Since its publication, there have been no modifications to the risk categories or to the CTX values. Further validation of this data and repeated multicentre studies with large sample sizes would be of significant clinical value.
CTX Debate
Despite the use of CTX for predictive purposes, a growing number of dissenting authors have questioned and even refuted the value of CTX as a predictive biomarker for BRONJ. The limitations of CTX include interpatient variability, nonstandardized laboratory reference ranges, variation in terms of fasting or non-fasting sampling, and low interpretability of CTX values for oral and IV bisphosphonates. A review of the literature retained for this article yielded a number of studies advocating the use of CTX as a predictive test, whereas others concluded that CTX was not an adequate marker. Several authors minimized the value of CTX studies as an absolute indicator for risk assignment as outlined by Marx6 but also proposed a more generalized “risk zone” categorization for patients with CTX values less than 150 pg/mL.5,12
Results
Table 4 Results of previous studies examining predictive value of CTX testing
| Study | Positive correlation of CTX with BRONJ |
Negative correlation of CTX with BRONJ |
| Bagan et al.7 | X | |
| Borromeo et al.3 | X | |
| Fleisher et al.10 | X | |
| Flichy-Fernandez20 | X | |
| Kunchur et al.12 | X | |
| Kwon et al.18 | X | |
| Kwon et al.19 | X | |
| Lazarovici et al.5 | X | |
| Lee and Suzuki8 | X | |
| Marx et al.6 | X | |
| O’Connell et al.1 | X | |
| Oliver and Badr11 | Not reported | Not reported |
BRONJ = bisphosphonate-related osteonecrosis of the jaw, CTX = C-terminal cross-linking telopeptide assay.
Of the articles retained for this publication, 11 reported on the predictive correlation between CTX and the risk for BRONJ. Most of these studies found no evidence of a predictive correlation between CTX and BRONJ (Table 4). In one study examining CTX values for 23 patients exhibiting BRONJ,18 the authors noted lower CTX results among these patients and concluded that a positive correlation does exist. They recommended CTX testing and risk stratification for all patients taking bisphosphonates. Marx and colleagues,6 who pioneered the CTX risk table, emphasized the need for testing and reported positive predictive value for CTX. Kwon and colleagues19 reviewed CTX values for 18 patients with BRONJ and concluded that CTX values did correlate with BRONJ; however, they found that lower CTX values did not correspond to severity of BRONJ in terms of size and number of lesions.
In contrast, a comparison of 25 patients divided into BRONJ and control groups yielded no statistically significant correlations between CTX level and size and number of instances of BRONJ.7 In another review of 123 patients with a history of bisphosphonate use, 24 of the patients had CTX values below 150 pg/mL, and all had uneventful healing after dental surgery.10 A study of 50 patients who had oral or IV bisphosphonate use and dental surgery revealed no positive link between CTX and BRONJ.20 The authors concluded “that it is doubtful whether the serum CTX test is of help in determining osteonecrosis risk in patients treated with oral bisphosphonates.” A separate study involving 348 patients suggested “risk-zone” categorization for patients with CTX below 150 pg/mL and concluded that “the CTX test is not predictive of the development of [BR]ONJ for an individual patient.”12 A review of clinical guidelines for patients taking bisphosphonates recommended CTX testing but made no positive predictive attributions correlating CTX and BRONJ.3 An additional study reviewing 163 patients found that “CTX is not a valid preoperative test to accurately assess the level of risk of developing [BR]ONJ and is not indicated in the oral surgery patient.”8 Similarly, in a study of 23 patients and their CTX results, CTX demonstrated no value in predicting BRONJ.1 Finally, the proposed Cochrane protocol for prevention of BRONJ in users of bisphosphonates did not include any recommendation for CTX testing at all.11 Some authors, while agreeing that CTX values of less than 150 pg/mL significantly increase the risk for BRONJ with a high degree of confidence, maintain that CTX is not a definitive predictor of the development of BRONJ.5
It is apparent from these disparate results that there exists no clear agreement on the value of CTX as a biochemical marker for predicting development of BRONJ. The limitations of the studies reviewed included relatively low power because of small sample sizes and differences in interpretation related to IV versus oral administration of the drug. Only 3 of the studies reviewed recommended CTX as a marker for predicting BRONJ.
Discussion
A review of the evidence in the literature indicates no obvious consensus on the effectiveness of CTX as a marker for BRONJ. In fact, some researchers are examining alternatives to CTX that may prove more promising. Recent studies have shown that radiographic evidence of widening of the periodontal ligament may be more sensitive than CTX in predicting BRONJ,10 whereas others are examining different biochemical markers, such as serum osteocalcin.18 The literature indicates that no single marker reliably predicts BRONJ, and a combination of evidence must be analyzed to determine risk. CTX remains more reliable and less variable than N-telopeptide, bone-specific alkaline phosphate or parathyroid hormone,1,5,19 but treatment should not be determined by the result of a single test alone. Good clinical judgment and a thorough review of the medical history remain the most effective means of determining appropriate treatment. In particular, the route and duration of bisphosphonate therapy must be considered to properly interpret CTX results. The obvious danger is that patients with a history of bisphosphonate use will be labelled as having a high risk and, as such, may experience limited access to care because of clinicians’ reluctance to treat this patient population.
Currently, there exist many unanswered questions with respect to the value of CTX testing, including differences between fasting and non-fasting states and specific applicability of the test for patients who have received oral and/or IV bisphosphonates. As our understanding of the effects of these drugs continues to expand, further research is warranted to identify reliable tests that are both sensitive and specific in determining the risk of BRONJ.
THE AUTHOR
References
- O’Connell J, Ikeagwani O, Kearns G. A role for C-terminal cross-linking telopeptide (CTX) level to predict the development of bisphosphonate-related osteonecrosis of the jaws (BRONJ) following oral surgery? Ir J Med Sci. 2012;181(2)1-6. Epub 2010 Jan 6.
- Statement by Merck Regarding FOSAMAX® (alendronate sodium) and Rare Cases of Osteonecrosis of the Jaw [news release]. Mercks 2010 Mar 9. Available: http://www.mercknewsroom.com/news/statement-merck-regarding-fosamax-alendronate-sodium-and-rare-cases-osteonecrosis-jaw
- Borromeo GL, Tsao CE, Darby IB, Ebeling PR. A review of the clinical implications of bisphosphonates in dentistry. Aust Dent J. 2011;56(1):2-9. Epub 2010 Dec 22.
- Lam DK, Sandor GK, Holmes HI, Evans AW, Clokie CM. A review of bisphosphonate-associated osteonecrosis of the jaws and its management. J Can Dent Assoc. 2007;73(5):417-22.
- Lazarovici TS, Mesilaty-Gross S, Vered I, Pariente C, Kanety H, Givol N, et al. Serologic bone markers for predicting development of osteonecrosis of the jaw in patients receiving bisphosphonates.J Oral Maxillofac Surg. 2010;68(9):2241-7.
- Marx RE, Cillo JE Jr, Ulloa JJ. Oral bisphosphonate-induced osteonecrosis: risk factors, prediction of risk using serum CTX testing, prevention, and treatment. J Oral Maxillofac Surg. 2007;65(12):2397-410.
- Bagan JV, Jimenez Y, Gomez D, Sirera R, Poveda R, Scully C. Collagen telopeptide (serum CTX) and its relationship with the size and number of lesions in osteonecrosis of the jaws in cancer patients on intravenous bisphosphonates. Oral Oncol. 2008;44(11):1088-9. Epub 2008 Apr 8.
- Lee CY, Suzuki JB. CTX biochemical marker of bone metabolism. Is it a reliable predictor of bisphosphonate-associated osteonecrosis of the jaws after surgery? Part II: a prospective clinical study. Implant Dent. 2010;19(1):29-38.
- Khan AA, Sandor GK, Dore E, et al. Canadian consensus practice guidelines for bisphosphonate associated osteonecrosis of the jaw. J Rheumatol 2008;35(7):1391-7.
- Fleisher KE, Welch G, Kottal S, Crag RG, Saxena D, Glickman RS. Predicting risk for bisphosphonate-related osteonecrosis of the jaws: CTX versus radiographic markers. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;110(4):509-16. Epub 2010 Jul 31.
- Oliver R, Badr M. Interventions for the prevention of osteonecrosis of the jaws in patients receiving bisphosphonate therapy undergoing oral surgery (Protocol). Cochrane Database of Systematic Reviews 2010, Issue 2. Art. No.: CD008355. DOI: 10.1002/14651858.CD008355.
- Kunchur R, Need A, Hughes T, Goss A. Clinical investigation of C-terminal cross-linking telopeptide test in prevention and management of bisphosphonate-associated osteonecrosis of the jaws. J Oral Maxillofac Surg. 2009;67(6):1167-73.
- Khan AA, Rios LP, Sandor GK, Khan N, Peters E, Rahman MO, et al. Bisphosphonate-associated osteonecrosis of the jaw in Ontario: a survey of oral and maxillofacial surgeons. J Rheumatol. 2011;38(7):1396-402.
- Khan AA, Sandor GKB, Dore E, Morrison AD, Alsahli M, Amin F, et al. Bisphosphonate associated osteonecrosis of the jaw. J Rheumatol. 2009;36(3):478-90.
- Fosamax [package insert]. Merck & Co. Inc., Whitehouse Station, NJ; 2012. Available:
- http://www.merck.com/product/usa/pi_circulars/f/fosamax/fosamax_pi.pdf (accessed 2013 May 2).
- Zometa [package insert].Novartis Pharmaceuticals Corporation. East Hanover, NJ; 2012. Available: http://www.pharma.us.novartis.com/product/pi/pdf/Zometa.pdf (accessed 2013 May 2).
- Aredia [package insert].Novartis Pharmaceuticals Corporation. East Hanover, NJ; 2012.
- Available: http://www.pharma.us.novartis.com/product/pi/pdf/aredia.pdf (accessed 2013 May 2).
- Kwon YD, Ohe JY, Kim DY, Chung DJ, Park YD. Retrospective study of two biochemical markers for the risk assessment of oral bisphosphonate-related osteonecrosis of the jaws: can they be utilized as risk markers? Clin Oral Implants Res. 2011;22(1):100-5. Epub 2010 Oct 13.
- Kwon YD, Kim DY, Ohe JY, Yoo JY, Walter C. Correlation between serum C-terminal cross-linking telopeptide of type I collagen and staging of oral bisphosphonate-related osteonecrosis of the jaws. J Oral Maxillofac Surg. 2009;67(12):2644-8.
- Flichy-Fernandez AJ, Alegre-Domingo T, Gonzalez-Lemonnier S, Balaguer-Martinez J, Peñarrocha-Diago M, Jimenez-Soriano Y, et al. Study of serum CTX in 50 oral surgical patients treated with oral bisphosphonates. Med Oral Patol Oral Cir Bucal. 2012;17(3)e367-70.
- Quest Diagnostics. Collagen Type I C-Telopeptide (CTx). [Accessed 2012 Nov 7]. Available: http://www.questdiagnostics.com/testcenter/TestDetail.action?ntc=17406.
- U.S. Department of Health & Human Services. FDA U.S. Food and Drug Administration. FDA Approves Thalomid (thalidomide) to Treat Multiple Myeloma. [Last updated 05/11/2009; accessed 2013 Mar 7]. Available: "http://www.fda.gov/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CDER/ucm095651.htm?utm_campaign=Google2&utm_source=fdaSearch&utm_medium=website&utm_term=thalomid multiple myeloma&utm_content=1.
- Denz U, Haas PS, Wasch R, Einsele H, Engelhardt M. State of the art therapy in multiple myeloma and future perspectives. Eur J Cancer. 2006;42(11):1591-600. Epub 2006 Jul 3.
- Gieseler F. Pathophysiological considerations to thrombophilia in the treatment of multiple myeloma with thalidomide and derivates. Thromb Haemost. 2008;99(6):1001-7.
- American Dental Association Council on Scientific Affairs. Dental management of patients receiving oral bisphosphonate therapy: expert panel recommendations. J Am Dent Assoc. 2006;137(8):1144-50.
Case Vignette 1
An 82-year-old female with a history of multiple myeloma and bilateral knee replacement presented for dental care. Her medication history included 8 months of IV pamidronate in each of 2010 and 2011, for a total of 16 months of therapy. She was due to resume IV administration of the bisphosphonate in November 2011, one month after her presentation for dental care in October 2011. Her dental findings included previously endodontically treated tooth 21 with fracture at the gingival level (Fig. 1) and heavily decayed teeth 36 and 37, with subosseous extension of the decay (Figs. 2 and 3). She was advised that extractions would not be appropriate, given the risk of BRONJ. Her treatment needs included endodontic retreatment of 21, with levelling of the root tip to accommodate a removable partial denture, root canal treatment for 36 and 37, and placement of a resin-modified glass ionomer cap over the root tips. She was sent for CTX testing at a Quest Diagnostics facility, for which the result was 866 pg/mL. According to Marx’s risk table,6 she would be classified as being at low risk for BRONJ. While the CTX result of 866 pg/mL indicates high bone turnover, and therefore good reparative and/or regenerative potential, the extensive history of IV administration of bisphosphonate, and imminent resumption of this therapy, suggested that the relative risk for BRONJ remained at 6.7%–9.1%, as reported in the literature.3,12
The patient was given clindamycin 600 mg PO 1 hour before treatment. Root canal treatment was performed on tooth 21, with the Profile GT rotary system (Tulsa/Dentsply; Tulsa, OK) and warm vertical gutta-percha (Fig. 4). The retained root tips were capped with Fuji II LC CORE Material (GC America; Alsip, IL). A partial removable denture was fabricated to fit over the retained root tip 21. Decay was removed from teeth 36 and 37, which led to the complete removal of both crowns. Sodium hypochlorite was substituted with 2% chlorhexidine solution to avoid spillage into the oral cavity. Canals were cleaned and shaped with GT rotaries and obturated using Thermafil (Tulsa/Dentsply; Tulsa, OK) (Figs. 5 and 6). The patient reported complete relief of her symptoms at follow-up appointments 1 and 2 weeks later.
This case demonstrates that CTX values alone are insufficient to determine appropriate treatment. It was also imperative to consider the patient’s past and future IV use of bisphosphonate in assigning risk and determining prognosis. Determination of the patient’s risk of BRONJ in this case did not follow the risk stratification developed by Marx,6 (Table 3) as the patient would have been assigned a very low risk according to the table. The author believes that the treatment provided and the decision not to perform extractions were appropriate, despite the patient’s high CTX value.
 Figure 1. Preoperative image of tooth 21 fractured at the gum level.
Figure 1. Preoperative image of tooth 21 fractured at the gum level.
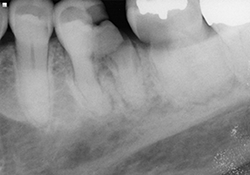
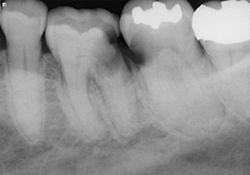
Figure 2 and 3. Preoperative radiographs showing teeth 36 and 37 with decay extending below the level of the bone.
 Figure 4 Completed endodontic retreatment of tooth 21 with resin cap.
Figure 4 Completed endodontic retreatment of tooth 21 with resin cap.
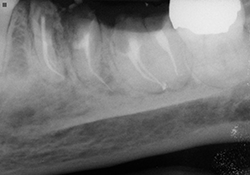
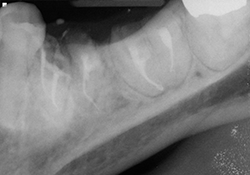
Figure 5 and 6. Completed root canals, with resin-modified glass ionomer caps.
Case Vignette 2
A 70-year-old male with a history of multiple myeloma presented for treatment in August 2010. His medication history included a 3-month course of IV zolendronate starting in August 2009. He had also been taking both methylprednisolone, a corticosteroid, and thalidomide, an immunomodulatory agent, twice a week. In 2006, the US Food and Drug Administration approved the use of thalidomide in conjunction with a corticosteroid in the treatment of multiple myeloma,22 with reported improvement in response rates of 60%–70% when combined with other chemotherapeutic agents.23,24 At the time of presentation, it had been approximately 9 months since discontinuation of the IV zolendronate. His dental findings were limited to mobility of his porcelain-fused-to-metal fixed partial denture 35-X-X-38. The crown on the distal abutment, tooth 38, had become decemented secondary to rampant decay, and a periapical lesion was visible on the radiograph. (Fig. 1) Under normal circumstances, he would have required the placement of multiple implants and ridge augmentation at sites 36 and 37, but these procedures were deemed to present too high a risk, on the basis of his medical history. The patient was sent for CTX testing at a local testing Quest Diagnostics facility, for which the result was 31 pg/mL, despite the patient having had a total of only 3 months’ exposure to IV bisphosphonate. The low CTX value would put him at high risk according to the established risk table (Table 3). A modified treatment plan was established, including sectioning of the bridge (leaving the mesial abutment 35 intact), extraction of tooth 38 and placement of a single implant, and fabrication of an implant-supported removable partial denture with a Locator (Zest Anchors; Escondido, CA) attachment. After sectioning of the bridge and surgical extraction of tooth 38, the socket was preserved with MinerOss (BioHorizons IPH; Birmingham, AL) an allograft mixture of cancellous and cortical bone chips, and secured with Mem-Lok collagen membrane (BioHorizons IPH; Birmingham, AL.) (Fig. 2). Four months later, a NobelReplace Tapered Groovy (Nobel Biocare; Zurich, Switzerland) 5 mm × 10 mm implant was inserted at 35 N/cm at site 38 (Figs. 3 and 4). Primary closure was obtained, and amoxicillin 500 mg PO tid for 1 week and a chlorhexidine 0.12% rinse bid were prescribed. The phase II uncovering of the implant took place 4 months later, and a 5 mm × 4 mm locator attachment was placed. Stability of the implant, measured with an Implant Stability Quotient meter (Osstell AB; Gothenburg, Sweden), was 74, stable enough for final restoration, according to the manufacturer’s instructions. An implant-supported removable partial denture was fabricated (Fig. 5), but the patient’s acceptance of the device was poor. Upon re-examination of the treatment plan, it was agreed to fabricate a fixed partial denture from implant 38 to tooth 35, using an Atlantis abutment (Atlantis Components; Cambridge, MA) (Figs. 6-8).
Unlike case vignette 1, in which the patient had a long history of IV administration of bisphosphonate and a relatively high CTX value, this patient had a low CTX value associated with a relatively short exposure to IV bisphosphonate therapy. On the basis of the expert panel recommendations for patients receiving bisphosphonate therapy (published in the Journal of American Dental Association in 2006),25 it was decided to proceed with a single implant placement, as the osseointegration of such implants tends to proceed well and there is no contraindication to their use in this patient population. Ridge augmentation and multiple implant placements were deemed to present too high a risk for BRONJ. The low CTX value may further be explained by the concurrent use of corticosteroids, which can contribute to lower bone remodelling activity. 6 Although the treatment provided can be viewed as a relatively higher-risk option, the author believes that a good clinical result was achieved by balancing the patient’s desire for a fixed prosthesis and the clinician’s limited treatment options based on the medical history. The author further believes that patients with low CTX values should not be indefinitely categorized as “high risk,” nor should dental practitioners refuse to treat these patients. In this case, through careful treatment planning and extensive communication between the patient and the dentist, a good outcome was achieved.
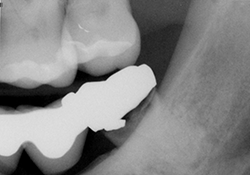 Figure 1. Preoperative radiograph of tooth 38 showing decay and periapical radiolucency.
Figure 1. Preoperative radiograph of tooth 38 showing decay and periapical radiolucency.
 Figure 2. Radiograph obtained 4 months after extraction showing good bone density and fill.
Figure 2. Radiograph obtained 4 months after extraction showing good bone density and fill.
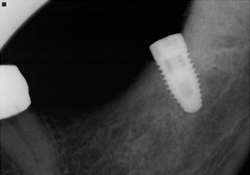
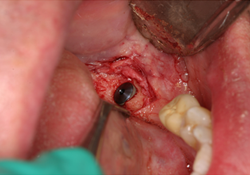
Figure 3 and 4. NobelReplace implant (5 mm × 10 mm) inserted at site 38.
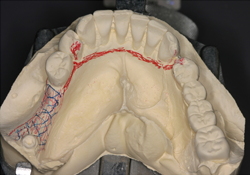 Figure 5. Design of the implant-supported removable partial denture on cast.
Figure 5. Design of the implant-supported removable partial denture on cast.
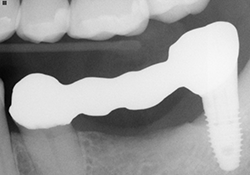 Figure 6. Fixed partial denture try-in on Atlantis abutment 38 to tooth 35.
Figure 6. Fixed partial denture try-in on Atlantis abutment 38 to tooth 35.
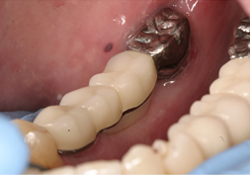
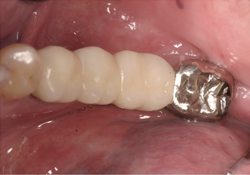
Figure 7 and 8. Photographs of the bridge cemented in place.

