Abstract
Direct thrombin inhibitors, specifically orally administered dabigatran etexilate, are emerging as alternatives to warfarin for anticoagulation in the management of atrial fibrillation and venous thromboembolism. The risk associated with bleeding events while taking dabigatran has been documented in multiple randomized controlled trials, but to date, no studies have focused on the risk of bleeding after dental extraction. Extraction of teeth is one of the most common surgical procedures and may cause significant bleeding, so a thorough understanding of the pharmacology of anticoagulant medications is required to prevent complications. With the increasing use of direct thrombin inhibitors, the safe management of patients taking these anticoagulants must be delineated. This review compares dabigatran and warfarin, especially in terms of their effects on dental and oral surgery practice, and examines best management of these patients in light of the existing literature.
Interest in the development of new anticoagulation medications is growing because of the limitations of parentally administered heparin and the interactions and monitoring concerns associated with vitamin K antagonists such as warfarin. Direct thrombin inhibitors constitute one class of medications that has been increasing in popularity for the prevention and treatment of venous thromboembolism and for the prevention of stroke in atrial fibrillation. This class of medication represents a new area for research, with multiple studies investigating both their efficacy and their safety for the treatment of these conditions.1-6 Particular interest has focused on dabigatran etexilate, an orally administered direct thrombin inhibitor. Dabigatran was approved by the US Food and Drug Administration (FDA) in October 2010 for the treatment of nonvalvular atrial fibrillation, and by August 2011, approximately 1.1 million prescriptions had been filled in the United States.7 Given the increasing use of this drug, it is important to consider its effect on the risk of surgical bleeding. So far, there have been no randomized controlled trials or case reports in the literature describing risk of bleeding or bleeding events related to minor oral surgery or dental procedures. The purpose of this article is to compare existing literature on direct thrombin inhibitors, specifically dabigatran etexilate (Pradaxa ® or Pradax ® ), with the literature on vitamin K antagonists, such as warfarin (Coumadin ® ), in terms of the risk of postoperative bleeding events, with the ultimate goal of establishing preliminary recommendations for clinicians until further research has been published to guide dental treatment.
Anticoagulation in the Dental Context
The extraction of teeth is one of the most commonly performed surgical procedures, and it is most often done in an outpatient setting. Although extraction is usually a minor procedure, patients who are at a higher risk of hemorrhage must be carefully assessed and proper measures for postoperative hemostasis undertaken. Over the past few decades, the primary treatment for long-term anticoagulation has been vitamin K antagonists such as warfarin.8 Although warfarin is widely used, as mentioned in the 2012 practice guidelines of the American College of Chest Physicians (ACCP),8 practitioners must be aware of certain concerns, including narrow therapeutic window, variability in dose response, and diet and drug interactions, as well as the requirements for good pharmacologic understanding, good patient communication and compliance with prescribed therapy.
Direct Thrombin Inhibitors
The coagulation protein factor II, also known as thrombin, is part of the common pathway of coagulation. Prothrombin is converted to thrombin by factor Xa and factor Va.9 The primary function of thrombin in coagulation is to convert fibrinogen to fibrin, a compound that is involved in the formation of clots.9 Thrombin is also involved in activating factors V, VIII, XI and XIII, as well as binding to thrombomodulin and activating protein C9 (Fig.1).
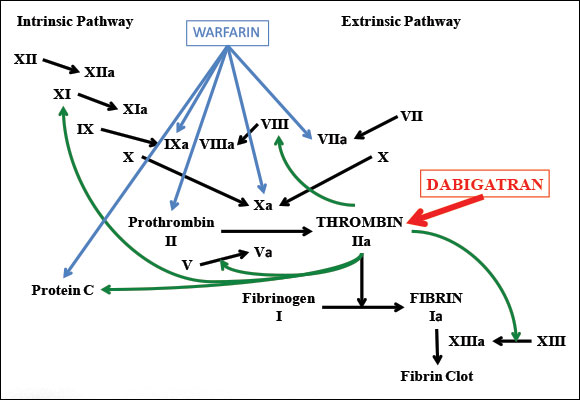 Figure 1: Coagulation cascade showing points of activity of warfarin (a vitamin K antagonist) and dabigatran (a direct thrombin inhibitor).
Figure 1: Coagulation cascade showing points of activity of warfarin (a vitamin K antagonist) and dabigatran (a direct thrombin inhibitor).
Direct thrombin inhibitors exert their effect through both the soluble and the fibrin-bound forms of thrombin.10 The FDA has approved 4 direct thrombin inhibitors for parenteral administration (lepirudin, desirudin, bivalirudin and argratroban) and 1 oral form (dabigatran).9 Oral direct thrombin inhibitors are of particular interest because they provide an attractive alternative to existing therapies such as parenteral heparins and vitamin K antagonists, such as warfarin. The advantages of direct thrombin inhibitors include a broad therapeutic window, fixed dosing and lack of interaction with cytochrome P450 enzymes; as such, they do not require the constant monitoring and dose adjustment that are needed for vitamin K antagonists, which in turn allows for more predictable anticoagulation.9 The prescription cost of dabigatran is significantly higher than that of warfarin, although research on the overall cost to the health care system has found that dabigatran is more cost-effective than warfarin.11 The parenteral direct thrombin inhibitors are primarily used for anticoagulation in patients with heparin-induced thrombocytopenia or those at risk of this condition.12 The oral medication dabigatran is currently approved by the FDA for use in nonvalvular atrial fibrillation. Dabigatran is not recommended for patients with prosthetic heart valves or hemodynamically significant rheumatic valvular heart disease.13 In Europe and Canada, it has also been approved for prophylaxis of venous thromboembolism. Dabigatran is approved for dosages of 75 mg and 150 mg bid; in Europe and Canada, a 110-mg formulation is also available.
Table 1 Pharmacologic data for dabigatran etexilate1-3,6,9
| Characteristic | Data for dabigatran etexilate |
| Class | Direct thrombin inhibitor |
| Doses available | 75 mg, 110 mg, 150 mg |
| Bioavailability | 3%–7% |
| Half-life | 11.5 hours |
| Time of peak plasma concentration | 2 hours |
| Routes of elimination | 80% renal, 20% hepatic |
| Indications | Atrial fibrillation, venous thromboembolism |
Dabigatran is poorly absorbed by the gastrointestinal tract, and the medication is therefore given as the prodrug dabigatran etexilate.9 This prodrug is completely converted to dabigatran immediately after ingestion.14 Peak plasma concentration is reached at approximately 2 hours, and the half-life is 11.5 hours in healthy individuals.14 Twenty percent of the absorbed drug undergoes hepatic metabolism, but the pharmacokinetic profile of dabigatran was not altered in patients with moderate hepatic impairment.14 Eighty percent of the drug is excreted unchanged via the renal system, and the dosage must be reduced for patients with renal insufficiency (creatinine clearance [CrCl] < 50 mL/min). Creatinine levels should also be considered if the medication is to be discontinued before a surgical procedure.6 The half-life increased to 16 hours in patients with CLCr between 30 and 50 mL/min and to 27 hours in those with CLCr less than 30 mL/min.6,9 The pharmacologic properties of dabigatran are summarized in Table 1. Adverse events associated with the use of dabigatran include dyspepsia, dizziness, dyspnea, peripheral edema, back pain, arthralgia, diarrhea and nasopharyngitis.1-4 However, all of these adverse events were more common among patients taking warfarin, with the exception of dyspepsia, which had an occurrence of approximately 11% in the dabigatran group.1 Dabigatran levels may be affected by other medications that affect the P-glycoprotein transporter, in particular ketoconazole, quinidine, verapamil, amiodarone, rifampicin and St. John’s wort.15
Risk of Bleeding
The development of an oral direct thrombin inhibitor has resolved many of the concerns about vitamin K antagonists, leading to dabigatran’s recent popularity. However, as with any new medication, practitioners must be cognizant of the potential for problems of various types. One area of concern with these medications is the treatment of complications of hemorrhage. A Cochrane review of 14 studies (total of 27 746 patients) comparing direct thrombin inhibitors with low-molecular-weight heparin (LMWH) and vitamin K antagonists showed no statistically significant difference in the risk of bleeding events, although the studies varied widely in their classification of bleeding.3 In the large multicenter Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) trial, which involved 18 113 patients who were taking dabigatran for atrial fibrillation, levels of both major and minor bleeding events were slightly lower among patients taking dabigatran, but the difference was not statistically significant.1 A secondary analysis of the RE-LY study16 showed that 4591 of the patients had undergone at least one invasive procedure for which oral anticoagulation was interrupted. Ten percent of these patients required dental procedures (the second most common procedure documented). The analysis showed similar rates of perioperative bleeding in association with both dosages of dabigatran (110 and 150 mg bid) and with warfarin, even among patients who had urgent or major surgery.16 More specifically, the incidence of major perioperative bleeding was 4.6% with warfarin, 3.8% with dabigatran 110 mg and 5.1% with dabigatran 150 mg.16
In spite of these findings, concerns have recently been raised with respect to severe bleeding events and their management among patients taking dabigatran.7,17 Existing approaches to monitoring of international normalized ratio (INR) and partial thromboplastin time (PTT) and to treatment of acute hemorrhage in patients taking either a vitamin K antagonist or heparin have been extensively studied, and the pharmacology of these agents is well known.8,12 Good results have been obtained with administration of vitamin K for mild INR elevation and with administration of prothrombin complex concentrates in cases of acute hemorrhage.8 Among patients receiving heparin, good reversal characteristics have been obtained with protamine sulphate.12
The tests used to determine anticoagulation levels in patients receiving dabigatran include activated PTT, thrombin time and ecarin clotting time; INR is less useful, as dabigatran has little effect on INR levels.6 Activated PTT provides a qualitative indication of anticoagulation by dabigatran but is not suitable for precise quantification of effect, especially at high concentrations.6 Thrombin time has a linear dose–response relationship with dabigatran, but current laboratory tests for determining thrombin time are not well standardized.6 Ecarin clotting time allows direct measurement of the activity of direct thrombin inhibitors, but this test is not widely used.6 Notably, unlike vitamin K antagonists and heparins, direct thrombin inhibitors lack a specific reversal agent.6 Recent investigations have assessed the effect of prothrombin complex concentrates on dabigatran therapy, with results showing no effect on any of the 3 tests (activated PTT, thrombin time or ecarin clotting time).18
Current treatments for patients who are taking dabigatran and who experience minor bleeding events involve delaying the next dose or discontinuing the drug.6 For moderate or severe bleeding, treatments include mechanical compression, surgical intervention, fluid replacement, hemodynamic support, oral charcoal application (if recent ingestion of dabigatran) and hemodialysis.6 For life-threatening bleeding, treatment includes administration of prothrombin complex concentrates or charcoal, in addition to supportive measures.6 The lack of a proven effective reversal strategy for dabigatran is an area of concern in emergency or severe hemorrhage situations.18
In studies comparing warfarin and dabigatran, the target INR was between 2.0 and 3.0, although this target was achieved in only 50% to 76% of patients.1-3 The management of patients receiving a vitamin K antagonist who require dental extractions has been thoroughly investigated.19-24 In a large multicentre trial of bleeding events following extractions (n = 900 patients), the incidence of bleeding was 1.55% among patients who were receiving a vitamin K antagonist along with local hemostatic measures and 0.89% in the control group (not statistically significant).20 In that study, the use of local hemostatic measures, such as suturing, gelatin sponge or cellulose mesh, with tranexamic acid mouth rinse reduced the risk of postoperative bleeding among patients taking a vitamin K antagonist to levels comparable to those observed in individuals without anticoagulation.20 In several studies, the use of tranexamic acid mouth rinse greatly reduced the risk of postoperative hemorrhage in patients receiving anticoagulation therapy.19-25
The recent ACCP practice guidelines for perioperative management of antithrombotic therapy offer a thorough review of the relevant literature regarding management of patients receiving vitamin K antagonist who require dental extractions.19 The guidelines conclude that continuing vitamin K antagonist therapy around the time of a minor dental procedure, with concurrent administration of an oral prohemostatic agent (e.g., 5 mL tranexamic acid rinse 5 to 10 minutes before surgery and 3 or 4 times daily for the next day or two), is associated with a low risk (< 5%) of a major bleeding event.19 Other studies have recommended the use of tranexamic acid for a longer postoperative period (5–7 days).20-23 When medically safe, partial interruption of vitamin K antagonist (for 2 or 3 days) was also an acceptable alternative, leading to an INR of 1.6 to 1.9 on the day of the procedure.19 The ACCP recommendations19 are comparable to those of the British Society of Haematology,25 which states that anticoagulation need not be stopped for dental extractions in patients with INR below 3.0. With the very low risk of a significant bleeding event in association with dental extractions and the ability to treat any events that do arise, the risks associated with discontinuing vitamin K antagonist therapy are of greater concern than the risk of a bleeding event.
Recommendations
To date, there have been no published studies investigating the incidence of bleeding events among patients receiving a direct thrombin inhibitor and requiring dental extractions. As this class of medication is prescribed for more patients, further data will become available, but for now, available research and evidence on related topics must be used to guide patient management.
All of the studies involving dabigatran that were reviewed showed a risk of hemorrhage statistically similar to that of warfarin when INR was between 2.0 and 3.0, with the exception of gastrointestinal bleeding which was slightly higher in the dabigatran group; in addition, some studies showed a lower incidence of intracranial, life-threatening, surgical site and minor bleeding with dabigatran.1-6,16 As such, for patients who are undergoing dental procedures or minor oral surgery procedures, such as simple extractions, it can be assumed that the risk of a major bleeding event will be low, similar to that of a patient with INR between 2.0 and 3.0. The risk of bleeding in association with surgical extractions, although increased, is also expected to be low, provided appropriate local hemostatic measures are applied. For patients who require multiple surgical extractions or significant oral maxillofacial surgical procedures, caution must be exercised and consideration given to discontinuing the direct thrombin inhibitor before the procedure, with timing determined by renal function. It is advised that all patients receiving such medications and undergoing dental extractions receive local hemostatic measures, including suturing, gelatin sponge, cellulose mesh and oral 4.8% tranexamic acid rinse qid for 2 to 5 days, as these measures are of considerable importance in controlling postoperative bleeding events.19-23 For patients who require extensive oral surgery in whom it is safe to stop the anticoagulant medication, renal function must be used to determine the appropriate duration of discontinuation.6
Recommendations for discontinuation of dabigatran in cases of standard risk of bleeding are 24 hours before the procedure for patients with CLCr above 50 mg/mL, 2 days beforehand for those with CLCr between 30 and 50 mg/mL and 2 to 5 days beforehand for those with CLCr less than 30 mg/mL.6 For procedures with a high risk of bleeding, discontinuation should occur 2 to 4 days before the procedure for patients with CLCr above 50 mg/mL, 4 days beforehand for those with CLCr between 30 and 50 mg/mL and more than 5 days beforehand for those with CLCr less than 30 mg/mL.6 If discontinuation of anticoagulation is not advisable and extensive major oral surgery procedures are required, bridging to an appropriate dose of subcutaneous LMWH is recommended, as the ACCP guidelines suggest for patients receiving vitamin K antagonists.8 Table 2 compares dabigatran with other commonly used anticoagulation and antiplatelet medications, in terms of mechanisms of action, recommendations for procedures and reversal agents.
Table 2 Common anticoagulation and antiplatelet medications taken by patients seen in dental offices
| Characteristic | Dabigatran | Warfarin | Heparin/LMWH | ASA | Clopidogrel |
| Mechanism of action | Direct thrombin (factor II) inhibitor | Vitamin K antagonist; inhibits factors II, VI, IX, X | Antithrombin III activator | Irreversible inhibition of thromboxane A2, inhibition of platelet aggregation | Blocks ADP receptor to prevent platelet aggregation |
| Approach for dental or minor surgical procedures | Perform procedure as long as possible after last dose; local hemostatic measures* | Ensure INR < 3.0; local hemostatic measures* | Hold morning dose; local hemostatic measures* | Local hemostatic measures* | Local hemostatic measures* |
| Approach for major surgical procedures | Discontinue 2–3 half-lives before surgery; adjust for renal function | Discontinue 5 days before surgery, bridge to LMWH or heparin | Last dose 24 h before surgery, resume next day | Discontinue 7–10 days before surgery, restart next morning or 24 h after surgery | Discontinue 7–10 days before surgery, restart next morning or 24 h after surgery |
| Reversal agents | None | Vitamin K, plasma products (FFP, PCC) | Protamine sulphate | Platelet transfusion | Platelet transfusion |
ADP = adenosine triphosphate, ASA = acetylsalicylic acid, FFP = fresh frozen plasma, INR = international normalized ratio, LMWH = low-molecular-weight heparin, PCC = prothrombin complex concentrates.
* Local hemostatic measures include suturing, gelatin sponge or cellulose mesh, along with oral 4.8% tranexamic acid rinse qid for 2–5 days.
Given the rapid onset of action of dabigatran (2 hours) and its relatively short half-life (11.5 hours), it is recommended that procedures be performed as late as possible after the most recent dose. It may also be feasible, if deemed medically safe by the patient’s physician, to consider omitting one dose before an appointment for minor procedures with a suspected higher risk of bleeding. This suggestion is based solely on pharmacologic data for dabigatran, and futher randomized controlled trials are required to confirm the appropriateness of this approach.
It is important to remember the mechanism of action and potential interactions of dabigatran when prescribing postoperative analgesics. In particular, dabigatran levels are not affected by opioid medications or by diclofenac (a nonsteroidal anti-inflammatory drug [NSAID]).9 Although dabigatran may not directly interact with NSAID medications, both types of drug increase the risk of bleeding. It is therefore advisable to avoid (when possible) or to use caution in prescribing additional medications that increase the risk of bleeding, such as NSAIDs or acetylsalicylic acid for pain management.15 Acetaminophen and opioid medications are recommended alternatives for patients who are taking dabigatran.
In the management of any patient receiving anticoagulation or antiplatelet therapy, it is important to assess the risk of bleeding, to allow appropriate treatment planning and modifications to minimize the risk of complications. The availability of proven methods of decreasing postoperative bleeding events, using local measures and prohemostatic agents, has led to recommendations to continue anticoagulation therapy during the period around dental extractions.19-25 As the number of patients taking direct thrombin inhibitors increases, understanding of appropriate management will also improve. Further observational studies and randomized controlled trials are required to determine and define appropriate guidelines for management. Until then, practitioners must rely on existing studies and evidence to compare the risks and, in discussion with their patients, to determine acceptable treatment plans and modifications.
THE AUTHORS
References
- Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldren J, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139-51. Epub 2009 Aug 30.
- Schulman S, Kearon C, Kakkar AK, Mismett P, Schellong S, Eriksson H, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med. 2009;361(24):2342-52.
- Salazar CA, Malaga G, Malasquez G. Direct thrombin inhibitors versus vitamin K antagonists or low molecular weight heparins for prevention of venous thromboembolism following total hip or knee replacement. Cochrane Database Syst Rev. 2010(4):CD005981.
- Eikelboom JW, MBBS; Wallentin L,; Connolly SJ, et al. Risk of bleeding with 2 doses of dabigatran compared with warfarin in older and younger patients with atrial fibrillation:an analysis of the randomized evaluation of long-term anticoagulant therapy (RE-LY) Trial. Circulation. 2011;123(21):2363-72. Epub 2011 May 16.
- Eriksson BI, Dahl OE, Rosencher N, Kurt AA, van Dicjk CN, Frostick SP, et al. Oral dabigatran etexilate vs. subcutaneous enoxaparin for the prevention of venous thromboembolism after total knee replacement: the RE-MODEL randomized trial. J Thromb Haemost. 2007;5(11):2178-85.
- van Ryn J, Stangier J, Haertter S, Liesenfeld KH, Wienen W, Feurig M, et al. Dabigatran etexilate – a novel, reversible, oral direct thrombin inhibitor: interpretation of coagulation assays and reversal of anticoagulant activity. Thromb Haemost. 2010;103(6):1116-27. Epub 2010 Mar 29.
- FDA U.S. Food and Drug Administration. FDA Drug Safety Communication: Safety review of post-market reports of serious bleeding events with the anticoagulant Pradaxa (dabigatran etexilate mesylate). [Internet] 2011 Dec 7 [cited 2012 Jan 14]. Available from: http://www.fda.gov/Drugs/DrugSafety/ucm282724.htm.
- Ageno W, Gallus AS, Wittkowsky A, Crowther M, Hylek EM, Palareti G, et al. Oral anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e44S-e88S.
- Lee CJ, Ansell JE. Direct thrombin inhibitors. Br J Clin Pharmacol. 2011;72(4):581-92.
- Bates SM, Weitz JI. The mechanism of action of thrombin inhibitors. J Invasive Cardiol. 2000 Dec;12 Suppl F:27F-32.
- Sorensen SV, Kansal AR, Connolly S, Peng S, Linnehan J, Bradley-Kennedy C, et al. Cost-effectiveness of dabigatran etexilate for the prevention of stroke and systemic embolism in atrial fibrillation: a Canadian payer perspective. J Thromb Haemost. 2011;105(5):908-19.
- Garcia DA, Baglin TP, Weitz JI, Samama MM, American College of Chest Physicians. Parental anticoagulants: Antithrombotic Therapy and Prevention of Thrombosis 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e24S-e43S.
- Health Canada. Recalls & alerts. Pradaxa (dabigatran, previously called Pradax): not to be used in patients with artificial heart valves; 2012 Dec 22 [cited 2013 Apr 25]. Available from: http://www.healthycanadians.gc.ca/recall-alert-rappel-avis/hc-sc/2012/16387a-eng.php
- Stangier J, Stähle H, Rathgen K, Roth W, Shekari-Nejad K. Pharmacokinetics and pharmacodynamics of dabigatran etexilate, an oral direct thrombin inhibitor, are not affected by moderate hepatic impairmentJ Clin Pharmacol. 2008;48(12):1411-9. Epub 2008 Sep 30.
- Dabigatran etexilate mesylate Product Monograph, Boehringer Ingelheim Pharmaceuticals, Inc. Ridgefield, CT 06877, 2011
- Healey JS, Eikelboom J, Douketis J, Wallenti L, Oldgren J, Yang S, et al. Periprocedural bleeding and thromboembolic events with dabigatran compared with warfarin results from the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) randomized trial. Circulation. 2012;126(3):343-8. Epub 2012 Jun 14.
- Cotton BA, McCarthy JJ, Holcomb JB. Acutely injured patients on dabigatran. [Letter]. N Engl J Med. 2011;365(21):2039-40.
- Eerenberg ES, Kamphuisen PW, Sijpkens MK, Meijers JC, Buller HR, Levi M. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo-controlled, crossover study in healthy subjects. Circulation. 2011;124(14):1573-9.
- Douketis JD, Spyropoulos AC, Spencer FA, Mayr M, Jaffer AK, Eckman MH, et al. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e326S-50S.
- Bacci C, Maglione M, Favero L, Perini A, Di Lenarda R, Berengo M, et al. Management of dental extraction in patients undergoing anticoagulant treatment Results from a large, multicentre, prospective, case-control study. Thromb Haemost. 2010;104(5):972-5.
- Ramström G, Sindet-Pderesen S, Hall G, Blomback M, Alender U. Prevention of postsurgical bleeding in oral surgery using tranexamic acid without dose modification of oral anticoagulants. J Oral Maxillofac Surg. 1993;51(11):1211-6.
- Borea G, Montebugnoli L, Capuzzi P, Magelli C. Tranexamic acid as a mouthwash in anticoagulant-treated patients undergoing oral surgery. An alternative method to discontinuing anticoagulant therapy. Oral Surg Oral Med Oral Pathol. 1993;75(1):29-31.
- Sindet-Pedersen S, Ramström G, Bernvil S, Blomback M. Hemostatic effect of tranexamic acid mouthwash in anticoagulant-treated patients undergoing oral surgery. N Engl J Med. 1989;30;320(13):840-3.
- Wahl MJ. Myths of dental surgery in patients receiving anticoagulant therapy. J Am Dent Assoc. 2000;131(1):77-81.
- Baglin TP, Keeling DM, Watson HG. Guidelines on oral anticoagulation (warfarin): third edition – 2005 update. British J Haematol. 2006;132(3):277-85.



