Abstract
A woman undergoing orthodontic treatment presented with recession and reduced keratinized gingiva on teeth 31 and 41. The patient declined creation of a donor site for conventional autogenous connective soft tissue grafting and opted for an acellular dermal matrix soft tissue substitute for root coverage. Orthodontic treatment followed, and the patient returned for orthognatic surgery after 12 years. Long-term follow up revealed that root coverage remained stable over time and creeping attachment on both teeth was observed. Unexpectedly, an increase in the width of keratinized gingiva was observed. No adverse effects of orthodontic treatment carried out after grafting were observed.
Periodontal plastic surgery is routinely used to treat soft tissue defects, and the technical basis for managing gingival recession has improved over the years. These procedures are now highly predictable and stable, leading to pleasant esthetic outcomes. Biomaterials, either soft tissue graft substitutes1,2 or agents that enable periodontal regeneration,3 have become important tools in improving outcomes as well as obviating the need for autogenous grafts. In some instances, biomaterials may also be convenient when anatomic concerns regarding the palatal donor area may hamper the harvest of adequate amounts of connective tissue to correct soft tissue defects.
Acellular dermal matrix (ADM) is a soft tissue graft substitute that has been deemed an invaluable biomaterial for skin4 and oral soft tissue reconstructions.5 Derived from human skin, a stringent process is used to render it sterile and devoid of viral and bacterial contamination. Cells are absent from ADM, but extracellular proteins of the connective tissue and basement membrane remain in the material, which, once grafted, is incorporated and remodeled by cells of the recipient area.6 ADM has been shown to be efficient and predictable in soft tissue reconstruction in edentulous soft tissue ridge defects,5 as a dressing material in bone grafting surgery7 and for root coverage.8,9
The number of reports on the long-term outcome of ADM grafting is limited; most follow-up periods range from 6 months to 2 years.8,9 Root coverage achieved with ADM seems to be stable, although changes seem to occur over time. In this article, we report on gingival recession in the lower anterior sextant in a patient undergoing orthodontic treatment who was treated with ADM 12 years earlier.
Case Report
A 27 year-old woman, with no reported systemic problems, presented to the periodontics clinic of the Pontifícia Universidade Católica do Rio Grande do Sul, Porto Alegre, Brazil, with the chief complaint of gingival recession affecting her lower central incisors. The patient was undergoing orthodontic treatment in preparation for orthognathic surgery. According to the orthodontist, only minor recession was visible before treatment, but recession at these sites had become more severe once orthodontic therapy had been initiated.
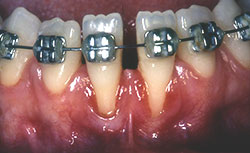
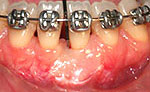
Clinical examination revealed 7-mm- and 5-mm-deep Miller's class III recession on teeth 31 and 41, respectively, with a probing depth of 3 mm at both sites. Widths of keratinized gingiva were 1 mm and 2 mm, respectively. The buccal–lingual dimension was also reduced, resulting in a thin periodontal biotype, and frenum insertion was high. The patient reported discomfort in response to cold and during brushing, which hampered adequate plaque control and caused mild gingivitis (Fig. 1). No pocketing was detected interproximally, as determined by clinical and radiographic examination. A relatively wide mesio-distal component between the incisors was evident because of the presence of a diastema.
Orthodontic treatment was discontinued during the periodontal treatment phase. The patient received full-mouth scaling and root planing and was given oral hygiene instructions. The choice of a connective tissue graft was rejected as anatomical issues at the palatal donor area hampered the harvesting of a graft of adequate size and thickness, and the patient refrained from creating an additional surgical donor area. Thus, an ADM option (AlloDerm, BioHorizons, Birmingham, AL, USA) was preferred.
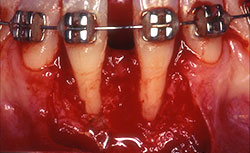
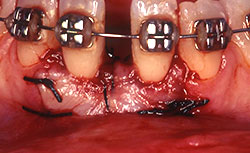
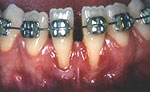
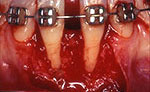
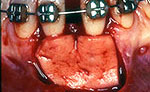
Before root coverage, frenectomy was performed to reduce soft-tissue pull between teeth 41 and 31, and the outcome was re-evaluated 2 months afterward. During the root coverage procedure, horizontal incisions were made at the level of the cemento-enamel junction (CEJ) and connected with vertical incisions down to the mucogingival junction (MGJ) distal to the target teeth. After intrasulcular incision, a partial-thickness flap was raised and care was taken to preserve the keratinized gingiva (Fig. 2). Gauze soaked in sterile saline was used to cover the wound while ADM material was prepared for grafting. A 2 cm × 4 cm piece of ADM was soaked in sterile saline for 10 minutes twice to ensure rehydration and then trimmed to cover the root and part of the surrounding periosteum. The material was placed with its connective tissue side facing the inner portion of the flap (Fig. 3) and sutured interproximally and laterally with a resorbable material (Vicryl Rapide 5-0, Johnson & Johnson, São Paulo, SP, Brazil). The flap was repositioned coronally, adjusted to cover the ADM material completely and then sutured (Ethicon Silk 4-0, Johnson & Johnson, São Paulo, SP, Brazil) (Fig. 4). A surgical dressing (COE-PAC, GC Corporation, Tokyo, Japan) was also applied, avoiding excessive pressure, and removed a week later. The patient was advised to rinse topically with chlorhexidine (PerioGard, Colgate-Palmolive, São Paulo, SP, Brazil) twice daily for 2 weeks, then return to the standard oral hygiene procedures, including brushing with a soft toothbrush and flossing. The patient was instructed to brush using the Stillman technique to avoid trauma to the tissue.
The patient postponed conclusion of the orthodontic treatment and periodontal maintenance consultations, returning only after 2 years. As shown in Figure 5, the root coverage procedure had been effective, but tooth 31 still showed a 1-mm recession bucally and mesially. The width of attached gingiva on tooth 31 had increased, although no significant changes had occurred on tooth 41. Although orthodontic treatment was successfully completed over the following 2 years as originally planned, the patient, once again, had to stop treatment; she declined orthognathic surgery and did not enroll in a regular periodontal maintenance program.
After another hiatus — 12 years after root coverage surgery — the patient returned to the clinic, now willing to undergo the originally planned orthognathic surgery. She was immediately referred to the department of periodontology for re-evaluation. Clinical examination showed that conditions were adequate, despite minor plaque deposits and bleeding on probing (Fig. 6). Completion of the orthodontic treatment 8 years earlier, which involved diastema closure, had no adverse effects on root coverage and no pockets were detected at the graft sites. Creeping attachment had occurred on both teeth, especially on the buccal and mesial aspects of tooth 31, and the buccal gingiva of tooth 41 appeared to be thicker.
NOTE: Click to enlarge images
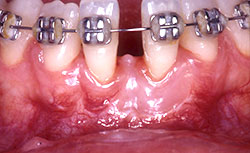 Figure 5. Two years after the root coverage procedure, a gain in the width of keratinized tissue can be seen, especially on the buccal aspect of tooth 31; the cemento-enamel junction is still slightly exposed.
Figure 5. Two years after the root coverage procedure, a gain in the width of keratinized tissue can be seen, especially on the buccal aspect of tooth 31; the cemento-enamel junction is still slightly exposed.
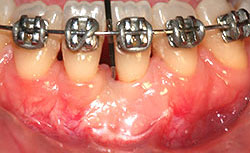 Figure 6. Twelve years after the root coverage procedure, creeping attachment of the buccal and mesial gingiva of tooth 31 was apparent, probably aided by the orthodontic treatment. The buccal gingiva of tooth 41 showed increased thickness. Reduced probing depth and slight bleeding were observed with some calculus formation.
Figure 6. Twelve years after the root coverage procedure, creeping attachment of the buccal and mesial gingiva of tooth 31 was apparent, probably aided by the orthodontic treatment. The buccal gingiva of tooth 41 showed increased thickness. Reduced probing depth and slight bleeding were observed with some calculus formation.
Discussion
Studies over the last 10 years have provided sound evidence that ADM is a suitable material for soft tissue reconstruction. It can be used in a partly5 or totally7 submerged fashion with satisfactory, stable results. It eliminates the need for additional surgical sites and problems associated with donor areas that may limit the amount of connective tissue available, especially in the treatment of multiple recessions. Long-term observational studies focusing on ADM outcomes are scarce. A 2-year follow up report showed that ADM may not be as effective as subepithelial connective tissue grafts in terms of root coverage; however, in a fairly significant number of cases, results improved over time.9 In the case described here, the gain in clinical attachment was stable; no additional recession or pocketing was detected, even though the subject had not enrolled in a regular periodontal maintenance program. Completion of orthodontic treatment caused no adverse effects on the gains achieved with ADM, and creeping attachment on both teeth led to full coverage of the roots.
Most studies report that root coverage with ADM can only produce a discrete increase in the height of keratinized gingiva, but it is possible that more significant changes may occur over longer periods. Hirsch et al.10 evaluated 262 teeth, in which recession was treated with ADM, and reported a mean approximate increase of 2 mm in keratinized gingiva after 2 years. ADM has also been shown to promote increases in soft tissue thickness after 2 years compared with coronally advanced flaps.1
Others have reported less favourable results of ADM treatment with regard to the height of keratinized gingiva and stability of the gain.11 The presence of a band of thick keratinized attached gingiva can play a role in gingival margin stability,12,13 and, in our case, root coverage resulted in reduced root sensitivity. This likely contributed to the hygiene status of our patient, as periodontal conditions were not significantly compromised even after a long period without professional periodontal maintenance care.
A recent report presented similar results in an upper canine, where a creeping attachment of 2 mm was observed after 10 years.14 In contrast, 2 teeth were involved in our case and they were subjected to orthodontic treatment. Therefore, a positive effect of tooth repositioning on the soft tissue changes we observed cannot be ruled out, as suggested by others.15,16
ADM is incorporated into the recipient tissues and progressively remodeled by native tissue.17 In the early healing stages, there is intense cell migration, especially of fibroblasts, and vascular growth, which sustains tissue organization within the protein framework provided by the material.18 No major inflammatory or immune events are observed, as ADM is devoid of cells and, thus, non-immunogenic; it is also likely that trace amounts of antibiotics present on the material may reduce bacterial growth.5 Even after longer healing periods, elastin fibres are still present within the grafted gingival tissue, suggesting that remnants of the protein framework persist.19 No new attachment is expected to occur on the denuded root surface; rather, fibrous apposition and increased tissue tonus offer resistance to probing.19 It has been suggested that ADM lacks the ability to induce differentiation of epithelium when used as a free gingival graft.17
It is not clear how the protein framework provided by ADM affects the recipient tissue bed on a long-term basis when ADM is used as a subepithelial graft. Thus, some induction of resident cells at the receptor site by ADM proteins toward a keratinizing phenotype could also have an impact on the outcome. Also noteworthy: the potential effects of factors related to the tissue donor and product lot cannot be ruled out, as demonstrated for other human-derived tissue substitute biomaterials.20
Orthodontic treatment effects on the position and keratinization of gingival margins have been suggested. Some reports suggest that orthodontic treatment can lead to increased keratinized buccal tissue.21,22 Nevertheless, according to others, even after retrusion of maxillary teeth, no major changes are expected to occur.23 In fact, the type of movement can play a role, as controlled extrusion leads to an increase in the height of keratinized gingiva.24
Furthermore, in young patients, the long-term result of an orthodontic approach involving intrusion and trauma elimination can lead to significant creeping attachment and root coverage.15 Therefore, considering the results reported by others and the course of events in our case, it is likely that orthodontic treatment played a role in the final outcome observed. Nevertheless, diastema closure did not seem to affect interproximal soft tissues, despite findings by others who reported beneficial effects of orthodontic alignment of teeth regarding papilla height.25
This case report suggests that many factors may play a role in the outcome of treatment with ADM: both subject-specific factors and material- or technique-related issues. Although not predictable, the changes observed after the soft tissue reconstruction described here were stable and seemed to improve over time. Long-term follow-up studies with a large number of patients are necessary to better characterize the ADM response.
| Gallery of all Figures in article | ||
 |
 |
 |
 |
 |
 |
THE AUTHORS
References
- de Queiroz Côrtes A, Sallum AW, Casati MZ, Nociti FH Jr, Sallum EA. A two-year prospective study of coronally positioned flap with or without acellular dermal matrix graft. J Clin Periodontol. 2006;33(9):683-9.
- Tal H, Moses O, Zohar R, Meir H, Nemcovsky C. Root coverage of advanced gingival recession: a comparative study between acellular dermal matrix allograft and subepithelial connective tissue grafts. J Periodontol. 2002;73(12):1405-11.
- Castellanos A, de la Rosa M, de la Garza M, Caffesse RG. Enamel matrix derivative and coronal flaps to cover marginal tissue recessions. J Periodontol. 2006;77(1):7-14.
- Chen WF, Barounis D, Kalimuthu R. A novel cost-saving approach to the use of acellular dermal matrix (AlloDerm) in postmastectomy breast and nipple reconstructions. Plast Reconstr Surg. 2010;125(2):479-81.
- Batista EL Jr, Batista FC, Novaes AB Jr. Management of soft tissue ridge deformities with acellular dermal matrix. Clinical approach and outcome after 6 months of treatment. J Periodontol. 2001;72(2):265-73.
- Núñez J, Caffesse R, Vignoletti F, Guerra F, San Roman F, Sanz M. Clinical and histological evaluation of an acellular dermal matrix allograft in combination with the coronally advanced flap in the treatment of Miller class I recession defects: an experimental study in the mini-pig. J Clin Periodontol. 2009;36(6):523-31.
- Batista EL, Jr, Batista FC. Managing soft tissue fenestrations in bone grafting surgery with an acellular dermal matrix: a case report. Int J Oral Maxillofac Implants. 2001;16(6):875-9.
- Harris RJ. Cellular dermal matrix used for root coverage: 18-month follow-up observation. Int J Periodontics Restorative Dent. 2002;22(2):156-63.
- Harris RJ. A short-term and long-term comparison of root coverage with an acellular dermal matrix and a subepithelial graft. J Periodontol. 2004;75(5):734-43.
- Hirsch A, Goldstein M, Goultschin J, Boyan BD, Schwartz Z. A 2-year follow-up of root coverage using sub-pedicle acellular dermal matrix allografts and subepithelial connective tissue autografts. J Periodontol. 2005;76(8):1323-8.
- Agudio G, Nieri M, Rotundo R, Cortellini P, Pini Prato G. Free gingival grafts to increase keratinized tissue: a retrospective long-term evaluation (10 to 25 years) of outcomes. J Periodontol. 2008;79(4):587-94.
- Kennedy JE, Bird WC, Palcanis KG, Dorfman HS. A longitudinal evaluation of varying widths of attached gingiva. J Clin Periodontol. 1985;12(8):667-75.
- Stetler KJ, Bissada NF. Significance of the width of keratinized gingiva on the periodontal status of teeth with submarginal restorations. J Periodontol. 1987;58(10):696-700.
- Santos A, Goumenos G, Pascual A, Nart J. Creeping attachment after 10 years of treatment of a gingival recession with acellular dermal matrix: a case report. Quintessence Int. 2011;42(2):121-6.
- Pini-Prato GP, Cozzani G, Magnani C, Baccetti T. Healing of gingival recession following orthodontic treatment: a 30-year case report. Int J Periodontics Restorative Dent. 2012;32(1):23-7.
- Zachrisson BU. Clinical implications of recent orthodontic-periodontic research findings. Semin Orthod. 1996;2(1):4-12.
- Harris RJ. Root coverage with a connective tissue with partial thickness double pedicle graft and an acellular dermal matrix graft: a clinical and histological evaluation of a case report. J Periodontol. 1998;69(11):1305-11.
- Luczyszyn SM, Grisi MF, Novaes AB Jr, Palioto DB, Souza SL, Taba M Jr. Histologic analysis of the acellular dermal matrix graft incorporation process: a pilot study in dogs. Int J Periodontics Restorative Dent. 2007;27(4):341-7.
- Richardson CR, Maynard JG. Acellular dermal graft: a human histologic case report. Int J Periodontics Restorative Dent. 2002;22:21-9.
- Schwartz Z, Somers A, Mellonig JT, Carnes DL Jr, Dean DD, Cochran DL, et al. Ability of commercial demineralized freeze-dried bone allograft to induce new bone formation is dependent on donor age but not gender. J Periodontol. 1998;69(4):470-8.
- Coatoam GW, Behrents RG, Bissada NF. The width of keratinized gingiva during orthodontic treatment: its significance and impact on periodontal status. J Periodontol. 1981;52(6):307-13.
- Dorfman HS. Mucogingival changes resulting from mandibular incisor tooth movement. Am J Orthod. 1978;74(3):286-97.
- Busschop JL, Van Vlierberghe M, De Boever J, Dermaut L. The width of the attached gingiva during orthodontic treatment: a clinical study in human patients. Am J Orthod. 1985;87(3):224-9.
- Pikdoken L, Erkan M, Usumez S. Gingival response to mandibular incisor extrusion. Am J Orthod Dentofacial Orthop. 2009;135(4):432 e431-6; discussion 432-3.
- Kandasamy S, Goonewardene M, Tennant M. Changes in interdental papillae heights following alignment of anterior teeth. Aust Orthod J. 2007;23(1):16-23.