Abstract
Objective: To improve understanding of how patient-reported outcomes following radiation therapy for head and neck cancer may be influenced by factors beyond the local effects of the radiotherapy.
Methods: Initially, 50 patients with head and neck cancer who were scheduled to undergo radiation therapy consented to participate in this prospective observational study. The participants underwent an oral examination before commencement of therapy and twice weekly over the therapy period. The 33 participants who finished the therapy underwent one more examination 4 to 6 weeks after its completion. At each session, clinical signs of oral mucositis were recorded with clinician-based scoring tools, and participants completed a questionnaire based on a visual analogue scale to record the perceived degree of impairment of common oral functions caused by oral mucositis. The strength of the correlation between these signs and symptoms at various points throughout the study period was appraised using a linear mixed model with robust repeated measures. The study participants with the most extensive manifestations of oral mucositis but only minor pain and limited adverse effects on oral functions (n = 6) were contrasted with those who had limited mucositis but more severe pain and adverse effects (n = 7). In addition, study participants with poor to moderate correlations between signs and symptoms (n = 5) were contrasted with those who had very good correlations (n = 10). Simple bivariate tests were used for these comparisons.
Results: Correlations between various signs and symptoms at all time points varied markedly at the individual level. The characteristics of study participants in the 2 subcohorts defined by poor to moderate and very good correlations between signs and symptoms were comparable, except perhaps in terms of age (p < 0.05, t test). Similarly, the participants in the 2 subcohorts defined by high manifestation with minor complaints and vice versa did not differ with regard to the variables recorded.
Conclusion: Patients with head and neck cancer often report adverse effects of radiation-related oral mucositis on daily oral functions that are discordant with objective clinical findings. Patient-reported outcomes should be included in any interventional studies of oral mucositis, and trends over time should be analyzed within individuals, rather than between individuals.
Patient-reported outcomes and experiences can augment clinical data and may help in assessing the effectiveness of interventions in cancer care.1-4 In cancer clinical research, the use of patient-reported outcomes has been recommended for patients with prostate,5 ovarian,6 gynecologic,7 oesophageal8 or head and neck cancer,9 among other types. Patient reporting can be used to monitor symptoms such as oral pain, skin changes, dental health, dry mouth, taste, saliva quality and quantity, difficulties with swallowing and mouth-opening, shoulder disability or immobility, vocal problems (including hoarseness), social domains and functional domains.9 One symptom that develops during radiotherapy is oral mucositis, which can interfere with cancer treatment,10 lead to weight loss (due to non-intake of food)11 or even prompt cessation of treatment.12 A novel tool known as PROMS (Patient-Reported Oral Mucositis Symptom) addresses the extent to which oral mucositis impairs oral functions, including dysphagia and dysgeusia.13
Many challenges remain in terms of establishing acceptable methodologic approaches to patient-reported outcomes and their optimal implementation in cancer clinical research.14,15 A primary challenge lies in defining the most relevant patient-reported outcomes.16 A second challenge is that the outcomes reported by patients and by clinicians are often incongruous.17 Demonstrating a strong correlation between patient-reported outcomes and relevant clinical outcomes remains important. Equally important, however, is to understand why certain patients differ from the majority in terms of their particular patient-reported outcomes and whether they share some distinctive characteristics. A better understanding of their characteristics will likely lead to improvements in patient care and could also reinforce the justification for including the subjective experiences reported by study participants in prospective clinical cancer research.
In a recent cohort study of patients with head and neck cancer, the authors observed that oral and pharyngeal mucositis of differing severity developed in all participants during the course of the 6- or 7-week treatment period.18 The participants were monitored closely twice weekly throughout the full treatment period by an investigator who used various clinician-based assessment tools in conducting the intraoral examinations and who also collected information by questionnaire. At the group level, the signs of oral mucositis as appraised by the clinician, using a tool of the National Cancer Institute (NCI)19 and the Oral Mucositis Assessment Scale (OMAS),20 correlated well with patient-reported experiences of oral mucositis as appraised by the Patient-Reported Oral Mucositis Symptom (PROMS) tool.13 At the individual level, however, large disparities were recognized in the reported adverse effects on oral functions attributed to oral mucositis. These findings prompted the current investigation to further explore data collected in the course of that earlier study,18 in the hopes of identifying potential explanations for these variations.
The overall objective of these secondary analyses was to determine whether the outcomes reported by patients with head and neck cancer were possibly influenced by factors beyond the local toxic effects of radiotherapy. Potential modifying factors included cancer diagnosis, treatment regimen, age, sex, ethnicity, smoking history, earlier pain experience, mood, coping mechanisms and culture. Two pairs of participant subgroups were contrasted: first, those who reported high PROMS scores but had relatively few clinical findings against those with low PROMS scores and extensive clinical manifestations of oral mucositis; and second, those with poor to moderate correlations between observed signs and patient-reported experience of oral mucositis against those with very good correlations between signs and symptoms.
Methods
Main Study
The methods for this study have been described in detail elsewhere.18 In brief, a prospective single-cohort study was undertaken at the Princess Margaret Cancer Centre, Toronto, Canada. The objective was to appraise the merits of supplementing clinical assessments of oral mucositis with the PROMS instrument for patients with head and neck cancer undergoing radiotherapy with or without concurrent chemotherapy. Study approval was obtained in 2009 from the Research Ethics Board of the University Health Network (reference 09-0231-CE). An a priori power analysis to establish a rank correlation of 0.90 between patient-reported and clinician-observed data yielded a sample group of 20 study participants (a = 0.05% and power of 80%, 2-tailed correlations) (Sample Power software, SPSS Inc., Chicago, IL). In general, dentists recognize that the management of patients with head and neck cancer can be challenging, because of poor oral health behaviours and compliance problems,21,22 and other studies of this patient population have had dropout rates as high as 66%.23 Therefore, in expectation of a high dropout rate, the investigators recruited 50 participants, well beyond the 20 indicated by the power calculation.
For inclusion in this study, participants had to be at least 18 years of age and had to have a diagnosis of carcinoma in the head and neck region, with a minimum Karnofsky performance status score of 60%. All potential participants were scheduled to receive curative radiotherapy for their cancer, with a minimum prescribed radiation dose of 54 Gray (Gy). For some of the participants concurrent chemotherapy was also planned.
The 50 consenting participants underwent an oral examination at baseline before commencement of cancer therapy. Seven participants did not complete their cancer therapy, 3 received less than the minimum 54 Gy dose of radiation, and 7 discontinued participation in the cohort study primarily because of fatigue. The remaining 33 participants were examined clinically twice weekly over their course of radiotherapy (7-week course for 25 patients, 6-week course for 7 patients and 4-week course for 1 patient) and then once more 4 to 6 weeks after completion of the therapy. The most common diagnosis among the 33 participants who completed the whole study was cancer of the oropharynx, T stages 1 and 2 (Table 1).
| T stage*; no. of patients | ||||||
|---|---|---|---|---|---|---|
| Location | Total no. (%) | T0/Tx | T1 | T2 | T3 | T4 |
| *T stage categories are based on the Union for International Cancer Control staging system.24 | ||||||
| Oral cavity | 5 (15) | 1 | 1 | 1 | 1 | 1 |
| Oropharynx | 13 (39) | 1 | 3 | 4 | 3 | 2 |
| Salivary glands | 6 (18) | - | 1 | 2 | 2 | 1 |
| Other | 9 (27) | 4 | - | 2 | 1 | 2 |
| Total | 33 (100) | 6 | 5 | 9 | 7 | 6 |
All study participants received intensity-modulated radiation therapy. The most common dose was fractions of 2 Gy over 33 or 35 visits over 6 or 7 weeks, respectively. The field of radiation and volume of irradiated tissue varied depending on tumour location and TNM cancer stage.24 About half of the study participants received concurrent chemotherapy (n = 15, 45%).
Clinical Examination
Three clinician-based scoring tools were used to record clinical signs of oral mucositis: the clinical component of the NCI Common Terminology Criteria for Adverse Events, version 3 (NCI-CTCAE v. 3),19 the clinical component of the OMAS20 and a tool locally developed in Toronto and termed TOTAL-VAS-OMAS.13 In the NCI-CTCAE v. 3, the occurrence and severity of oral mucositis are graded with an ordinal score ranging between 0 (none) and 4 (most) as observed at any site within the oral cavity. The OMAS concept is based on scoring between 0 (none) and 3 (ulceration) or 2 (erythema) in 9 specific intra-oral locations. Hence, the maximum sum of scores is 27 (9 sites × score of 3) for ulceration and 18 (9 sites × score of 2) for erythema. The TOTAL-VAS-OMAS tool consists of 2 visual analogue scales (VASs) ranging from 0 to 100 mm for full-mouth assessments of erythema and ulceration, respectively. Before the study commenced, the scoring of clinical examiners was calibrated by having them score oral mucositis of various degrees of severity in laminated clinical photographs. These photographs were also used for periodic recalibration during the study period, to prevent "drifting" of intra-rater assessments.
Patient Questionnaire
At every clinical examination, each participant completed a PROMS questionnaire13 to appraise the degree of impairment caused by oral mucositis with regard to common oral functions. The PROMS scale consists of 10 questions, which the patient answers by setting a mark on a horizontal 100-mm line (the VAS). One question focused on mouth pain caused by oral mucositis, ranging from none to worst possible. Another question assessed dysgeusia, ranging from hypogeusia to complete loss of taste. The remaining 8 questions dealt with how much pain was caused by oral mucositis on the day of the clinical examination and its impact on various oral functions, including swallowing.
Statistical Analyses
Main Study
Spearman rank correlation values were determined between the PROMS scale values and, respectively, the NCI-CTCAE v. 3, the OMAS and the TOTAL-VAS-OMAS scores. A linear mixed model with robust repeated measures was used to appraise the strength of correlations at various time points throughout the observation period, taking into account the repeated nature of the measurements. A Bonferroni correction was applied to all statistical tests to account for multiple testing of the same measures. All of the multivariate statistical tests were performed by an independent professional statistician using the statistical procedures PROC CORR and PROC MIXED in the SAS System version 9.2 software (SAS Institute, Cary, NC). Correlations with a Spearman rho of less than 0.20 were considered poor, 0.21–0.40 fair, 0.41–0.60 moderate, 0.61–0.80 good and more than 0.80 very good.25
Secondary Analyses
The characteristics of study participants who had the most extensive manifestations of oral mucositis but reported only minor pain and adverse effects on oral functions (termed "stoical sufferers"; n = 6) were contrasted with those of patients with the most minor manifestations but reporting extensive pain and adverse impact on oral functions (termed "complaining sufferers"; n = 7). In addition, participants with poor to moderate correlations between clinical signs and patient-reported oral mucositis (n = 5) were contrasted with those with very good correlations (n = 10). Because the number of study participants was small in relation to the many identifiable variables, it was considered inappropriate to apply multivariate statistical analyses. Instead, simple bivariate tests were used, i.e., the Fisher exact or chi-squared test for categorical variables and the Student t test for age comparisons in the 4 identified subcohorts.
Results
All participants in this study experienced oral mucositis during the course of radiotherapy, which for some patients manifested as erythema after an absorbed dose of about 6 Gy, increasing thereafter in concert with increased absorption of therapeutic radiation. Some participants reported pain and impairment of oral functions in their first week of radiation treatment. For all patients over all time points, the measured correlations (expressed as Spearman rho) between clinician-determined scores and patients' experience of oral mucositis ranged between 0.65 and 0.75 (Table 2). The correlations were fairly consistent in the early, middle and late stages of radiotherapy, except for correlations between OMAS ulceration and PROMS scale values at early time points (Table 2). At the individual level, however, Spearman rho values varied markedly, indicating poor to moderate, to very good correlations, as exemplified by study participants "A" and "B" (Figs. 1 and 2, respectively). The characteristics of study participants in the 2 subcohorts defined by poor to moderate and very good correlations were comparable, except perhaps with regard to age (p = 0.038, t test) (Table 3).
Click to enlarge

Figure 1: Representative study participant with mostly poor to moderate correlations between clinical signs and self-reported experience of oral mucositis, as represented by individual components of the Patient-Reported Oral Mucositis Symptom tool (Spearman rho 0.16–0.70). NCI and NCI v. 3=National Cancer Institute Common Terminology Criteria for Adverse Events, version 3; OMAS=Oral Mucositis Assessment Scale; PROMS=Patient-Reported Oral Mucositis Symptom tool; Tx=treatment; VAS=visual analogue scale.
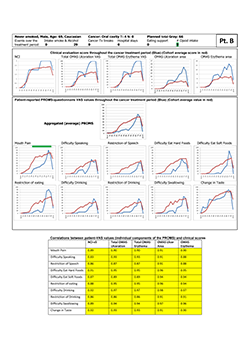
Figure 2: Representative study participant with very good correlations (dark yellow highlighting in tabular element) between clinical signs and self-reported oral mucositis experience, as represented by the individual components of the Patient-Reported Oral Mucositis Symptom tool (Spearman rho.0.83–0.98). NCI and NCI v. 3=National Cancer Institute Common Terminology Criteria for Adverse Events, version 3; OMAS=Oral Mucositis Assessment Scale; Tx=treatment; VAS=visual analogue scale.
| Comparator measure (vs. aggregate PROMS); Spearman rho* | |||||
|---|---|---|---|---|---|
| Cumulative dose of radiotherapy | NCI-CTCAE v. 3 |
TOTAL-VAS-OMAS ulceration | TOTAL-VAS-OMAS erythema | OMAS ulcer area | OMAS erythema area |
| NCI-CTCAE v. 3=National Cancer Institute Common Terminology Criteria for Adverse Events version 3,19 OMAS=Oral Mucositis Assessment Scale,20 PROMS=Patient-Reported Oral Mucositis Symptom, TOTAL-VAS-OMAS=locally developed tool based on visual analogue scale. *Spearman rho < 0.20=poor correlation, 0.21–0.40=fair correlation, 0.41–0.60=moderate correlation, 0.61–0.80=good correlation, > 0.80=very good correlation.25 |
|||||
| < 20 Gy | 0.51 | 0.25 | 0.54 | 0.24 | 0.54 |
| 20–60 Gy | 0.54 | 0.57 | 0.60 | 0.41 | 0.47 |
| > 60 Gy | 0.52 | 0.48 | 0.47 | 0.45 | 0.44 |
| Overall | 0.75 | 0.75 | 0.78 | 0.65 | 0.69 |
| Characteristic | Very good correlation (Spearman rho > 0.80) (n = 10) |
Poor to moderate correlation (Spearman rho ≤ 0.60) (n = 5) |
Remaining participants (n = 18) | Total no. (%) of patients (n=33) |
|---|---|---|---|---|
| SD=standard deviation. *Data missing for one or more patients. †64 and 60 Gy planned for these 2 patients. |
||||
| Sex | ||||
| Men | 9 | 4 | 12 | 25 (76) |
| Women | 1 | 1 | 6 | 8 (24) |
| Ethnicity | ||||
| Caucasian | 8 | 5 | 14 | 27 (82) |
| Other | 2 | 0 | 4 | 6 (18) |
| Age (years) | ||||
| Mean ± SD | 59 ± 8 | 68 ± 6 | 60 ± 12 | 61 ± 9 |
| Range | 49–70 | 62–78 | 39–80 | 39–80 |
| Dental status | ||||
| Good | 4 | 0 | 11 | 15 (45) |
| Fair to poor | 5 | 4 | 7 | 16 (48) |
| Edentulous | 1 | 1 | 0 | 2 (6) |
| Smoking status | * | (n=32)* | ||
| Never | 3 | 0 | 6 | 9 (28) |
| Ex-smoker | 4 | 2 | 10 | 16 (50) |
| Present smoker | 2 | 3 | 2 | 7 (22) |
| Alcohol use | * | * | (n=31)* | |
| No | 3 | 0 | 8 | 11 (35) |
| Yes | 6 | 5 | 9 | 20 (65) |
| Primary tumour location | ||||
| Oral cavity | 3 | 0 | 2 | 5 (15) |
| Oropharynx | 4 | 2 | 7 | 13 (39) |
| Salivary glands | 1 | 1 | 4 | 6 (18) |
| Other | 2 | 2 | 5 | 9 (27) |
| T stage | ||||
| T0 or T1 | 4 | 2 | 5 | 11 (33) |
| T2 | 1 | 1 | 7 | 9 (27) |
| T3 or T4 | 5 | 2 | 6 | 13 (39) |
| N stage | ||||
| N0 or N1 | 5 | 3 | 12 | 20 (61) |
| N2 | 4 | 2 | 6 | 12 (36) |
| N3 | 1 | 0 | 0 | 1 (3) |
| Planned dose of radiation (Gy) | ||||
| 70 | 5 | 3 | 13 | 21 (64) |
| 66 | 3 | 2 | 5 | 10 (30) |
| < 66 | 2 | 0 | 0 | 2 (6)† |
| Planned chemotherapy | ||||
| No | 6 | 4 | 8 | 18 (55) |
| Yes | 4 | 1 | 10 | 15 (45) |
The study participants in the 2 subcohorts defined by high manifestation and minor complaints and vice versa did not differ with regard to variables recorded (Table 4). In particular, the group of "stoical sufferers" (high manifestation, minor complaints) is exemplified by study participant "C" (Fig. 3). This 50-year-old white nonsmoking man experienced maximum clinical scores for oral mucositis yet, except for "difficulties eating hard food" and "change in taste," values on the PROMS scale were low during the full 6-week treatment period. Moreover, he reported no intake of opioid analgesics. Correlation between individual components of the PROMS tool and the clinician-determined scales was good to very good (Spearman rho 0.70–0.96), except for difficulties with and restriction of drinking and speech.
Study participant "D" (Fig. 4) is presented as an example of a "complaining sufferer." This 63-year-old white man had only modest manifestations of oral mucositis, yet reported near-maximum scores with the PROMS tool. He also reported high pain levels, despite use of opioids. He was a smoker and continued to smoke during the 6-week treatment period, although the number of cigarettes was reduced to 1 or 2 per day. Correlation between individual components of the PROMS tool and the clinician-determined scales was high (Spearman rho 0.76– 0.98) except for difficulties eating hard foods and change of taste.
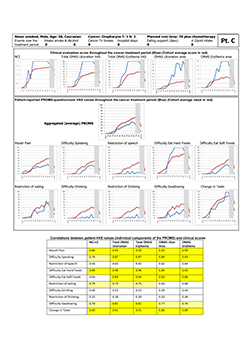
Figure 3: Representative "stoical sufferer," showing extensive manifestation of oral mucositis but reporting only minor pain and little adverse effect on oral functions. NCI and NCI v. 3=National Cancer Institute Common Terminology Criteria for Adverse Events, version 3; OMAS=Oral Mucositis Assessment Scale; PROMS=Patient-Reported Oral Mucositis Symptom tool; Tx=treatment; VAS=visual analogue scale. In tabular element, dark yellow highlighting indicates very good correlation between clinical signs and self-reported oral mucositis experience (Spearman rho > 0.80), and light yellow highlighting indicates good correlation (Spearman rho 0.61–0.80).
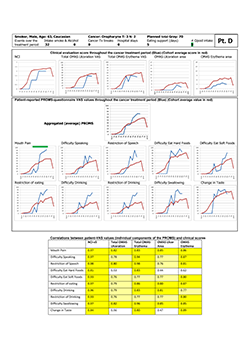
Figure 4: Representative "complaining sufferer," showing minor manifestation of oral mucositis but reporting extensive pain and adverse impact on oral functions. NCI and NCI v. 3=National Cancer Institute Common Terminology Criteria for Adverse Events, version 3; OMAS=Oral Mucositis Assessment Scale; PROMS=Patient-Reported Oral Mucositis Symptom tool; Tx=treatment; VAS=visual analogue scale. In tabular element, dark yellow highlighting indicates very good correlation between clinical signs and self-reported oral mucositis experience (Spearman rho > 0.80), and light yellow highlighting indicates good correlation (Spearman rho 0.61–0.80).
| Major OM, minor impact (n = 6) | Minor OM, major impact (n = 7) | Remaining participants (n = 20) | Total no. (%) of patients (n=33) |
|
|---|---|---|---|---|
| OM=oral mucositis, SD=standard deviation. *Data missing for one or more patients. †64 and 60 Gy planned for these two patients. |
||||
| Sex | ||||
| Men | 6 | 5 | 14 | 25 (76) |
| Women | 0 | 2 | 6 | 8 (24) |
| Ethnicity | ||||
| Caucasian | 5 | 6 | 16 | 27 (82) |
| Other | 1 | 1 | 4 | 6 (18) |
| Age (years) | ||||
| Mean ± SD | 63 ± 11 | 61 ± 9 | 61 ± 11 | 61 ± 9 |
| Range | 50–78 | 42–67 | 39–80 | 39–80 |
| Dental status | ||||
| Good | 3 | 4 | 8 | 15 (45) |
| Fair to poor | 2 | 2 | 12 | 16 (49) |
| Edentulous | 1 | 1 | 0 | 2 (6) |
| Smoking status | * | (n=32)* | ||
| Never smoked | 3 | 1 | 5 | 9 (28) |
| Ex-smoker | 2 | 5 | 9 | 16 (50) |
| Present smoker | 1 | 1 | 5 | 7 (22) |
| Alcohol use | * | (n=31)* | ||
| No | 1 | 4 | 6 | 11 (35) |
| Yes | 5 | 3 | 12 | 20 (65) |
| Primary tumour location | ||||
| Oral cavity | 1 | 1 | 3 | 5 (15) |
| Oropharynx | 3 | 2 | 8 | 13 (39) |
| Salivary glands | 0 | 2 | 4 | 6 (18) |
| Other | 2 | 2 | 5 | 9 (27) |
| T stage | ||||
| T0 or T1 | 1 | 3 | 7 | 11 (33) |
| T2 | 2 | 1 | 6 | 9 (27) |
| T3 or T4 | 3 | 3 | 7 | 13 (39) |
| N stage | ||||
| N0 or N1 | 3 | 5 | 12 | 20 (61) |
| N2 | 3 | 2 | 7 | 12 (36) |
| N3 | 0 | 0 | 1 | 1 (3) |
| Planned dose of radiation (Gy) | ||||
| 70 | 5 | 3 | 13 | 21 (64) |
| 66 | 1 | 4 | 5 | 10 (30) |
| < 66 | 0 | 0 | 2 | 2 (6)† |
| Planned chemotherapy | ||||
| No | 3 | 3 | 12 | 18 (55) |
| Yes | 3 | 4 | 8 | 15 (45) |
Discussion
From the results of this study, it is clear that clinical observations of oral ulceration can differ substantially from individual patients' experiences of oral mucositis. Thus, reliance upon clinical measures of oral ulceration and mucositis alone to gauge patients' symptoms (with regard to oral mucositis) following radiotherapy and/or chemotherapy must be reconsidered. In this small study, the characteristics of particular study participants were not clearly associated with discrepancies between clinician-observed signs and patient-reported symptoms. Only age was identified as differing between 2 subgroups defined in terms of poor to moderate vs. very good correlation between signs and symptoms over the cancer treatment period. That younger and older patients may respond differently to cancer treatment could be an indirect effect of dissimilar etiopathogenesis, with a likely higher prevalence of human papillomavirus–associated cancer in the younger age group. Failure to identify particular participant characteristics as being associated with discrepancies between clinician-observed signs and patient-reported symptoms may be an effect of a statistical type 2 error in this relatively small study sample. Alternatively, it may be the result of specific aspects of the design or execution of the study protocol. Specifically, the diagnostic abilities and perceptions of the clinical examiners under the given examination settings may have been inadequate. Alternatively, subepithelial tissue damage as a manifestation of oral mucositis may have been variable across the current study sample because of variations in treatment regimens. A third possibility is that patients differed with regard to responsiveness to a given level of tissue damage, pain or dysfunction. These 3 possibilities are discussed in more detail below. A mixed model analysis with a larger sample size may provide better data in this regard.
Examiners' Diagnostic Abilities
The calibrated clinical examiners used 2 dental mouth mirrors and a high-power head lamp as the light source for clinical assessment of oral mucositis. Although the standard routine was to undertake a structured examination of all intraoral and upper pharyngeal areas, pharyngeal oral mucositis might have been underdiagnosed in some cases if study participants were unable to fully open the mouth because of pain or trismus. However, participants with oropharyngeal cancer did not appear to be over- or under-represented in any of the subcohorts (Tables 3, 4). Study participant "D" (Fig. 4), who had oropharyngeal cancer, might have had oral mucositis that went undetected, as the examiner did not use an endoscope. Regardless, it bears repeating that mere clinical assessment of ulcers (e.g., measuring ulcer size) may be inadequate to determine the actual clinical effect of oral mucositis on any particular patient. This is important not only in relation to management of the cancer treatment itself, but also in the evaluation of potentially helpful therapeutic agents to prevent or ameliorate the severity of oral mucositis.
Variations in Treatment Regimens
Possible effects of radiation dose and concurrent chemotherapy did not explain the variation in reported adverse effects or poor correlations (Tables 3, 4). All study participants received the same intensity-modulated radiation therapy, even though the targets and consequently the fields of irradiation differed. Analysis of these dosimetric factors will be the subject of future work. Although some information is available regarding the relationship between tissue damage and dosage,26 the authors of the current work found no papers that studied a possible interdependency between tissue dosages and patient-reported pain. Some reports27,28 have indicated that chemotherapy combined with radiation treatment makes patients more susceptible to oral mucositis, whereas other investigators have reported no difference in this regard.29 In the current study, 45% of the study participants received chemotherapy, but under the conditions used here, it was not apparent that concomitant chemotherapy resulted in more or less pain and/or better or worse correlations between objective signs and subjective symptoms.
Variations in Patient Responsiveness
Individuals differ with regard to their responsiveness to a given amount of tissue damage, pain or dysfunction, and a reaction to oropharyngeal pain is likely linked to the local intraoral condition, the patient's general medical condition and his or her personality traits, boosted by support from family or close community. Certainly, it is well known that personality traits and even levels of cognitive function can alter perceptions of pain, as well as reactions and responses to treatments for pain.30
Given that a single ulceration site may cause just as much suffering as multiple and/or confluent areas, it is debatable as to what is the most meaningful approach to interpreting scores that originate from various scales that measure only clinical manifestations of oral mucositis. Moreover, sums of scores and average scores may be misleading if a few high scores are "neutralized" by sums of scores from multiple intra-oral sites. This statistical dilemma has been discussed by the developers of several scoring systems,20,31 but so far, no consensus has been reached.
A range of cofactors linked to lifestyle and medical comorbidity have been identified as risk factors for increased oral mucositis. Smoking has not been linked consistently with any particular presentation of oral mucositis, and it has been demonstrated as a risk factor for higher,32 lower33 or no34 effects on levels of oral mucositis, with no elaboration as to whether the pain and adverse effect on oral functions caused by the mucositis is amplified or diminished. The same applies to oral hygiene.35-38 It has been suggested that some individuals may be more susceptible to mucosal damage because of genotypic variation.39 The subcategory of oropharyngeal cancers linked to human papillomavirus, rather than traditional etiological factors, may also present with different symptoms during cancer treatment.40 This factor may be partially responsible for the identification of a significant difference in age between groups.
The current study aimed primarily to monitor closely the development of oral mucositis both clinically and in terms of patients' experiences, to describe the extent of any adverse effects that mucositis might have on various oral functions. In this regard, it was noted that several terms could be applied to characterize the experiences of patients with oral mucositis, including anxiety, distress, pain, exhaustion, fatigue and nausea. At the time the study was conceived, questionnaire burden was a concern, and it was therefore considered counterproductive to burden participants with additional questionnaires to address other functional issues (e.g., coping styles, level of distress, personality indices, comorbidity status or health-related quality-of-life inventories).
General Findings
Earlier pain experience and different coping mechanisms may also have influenced the way in which participants answered the PROMS questionnaire. An example is study participant "C" (Fig. 3), who commented to the investigator that he "was sure he was going to be fine" and continued to show a very optimistic attitude at all study appointments. In general, people with dispositional pessimism tend to report more pain than those who are optimistic.41,42 Moreover, for some of the participants, early experiences with acute, debilitating oral mucositis caused anxiety and may have led them to embellish reports of discomfort; however, as the therapy progressed, their perception of pain and adverse effects on oral functions became more tempered.
Individuals, whether participating in a trial or not, are influenced by mood and psychological status on the day they are asked to complete questionnaires.43,44 In many instances in the current study, participants' psychological status on the particular examination day appeared to influence their PROMS reporting. More than once, particularly toward the end of the treatment period, study participants expressed happiness to know that their radiotherapy sessions were coming to an end and, accordingly, it was noted (but not quantified, because the sample was not large enough) that these participants tended to record lower VAS values for the effect of oral mucositis during these last few study visits.
Many patients with newly diagnosed head and neck cancer experience high levels of mental distress and psychiatric morbidity during treatment.45,46 According to one estimate, about one-third of all patients appear to have a probable major mood disorder, with females appearing more anxious than males at the time of diagnosis, and patients under 65 years of age appearing more anxious than those over 64.47 It has also been noted that patients who experience oral mucositis show a significant increase in mood disturbance.48
Finally, different coping mechanisms49 may influence the way patients feel about the effects of oral mucositis and the way they report their symptoms on the PROMS questionnaire. Because there appears to be a relationship between anxiety and the use of negative coping styles,50 all patient-reported outcomes should be viewed with caution. Yet it is the patient-reported outcomes that should dictate management of a patient with oral mucositis, as opposed to management being based merely on the size, location or extent of lesions.
Ethnic51 or cultural52,53 differences may lead to differences in both reporting and coping with pain. The possible effect of cultural background was not studied in the current investigation. Most of the study participants were white (82%), but their cultural backgrounds may not have been the same.
It is often tempting to interpret patient-reported symptom data at the inter-individual level, rather than the intra-individual level. Some patients may enter a higher score than other patients depending on several factors including, but not limited to, previous experiences regarding illness or pain.18 However, one conclusion from the current study is that the most appropriate data for comparison may be measures of within-participant change before and after an intervention, as is advised when appraising quality-of-life improvements.54 This type of approach could optimize individual management of patients, as alluded to above. What remains to be resolved is identification of the relative intra-individual changes in patient-reported VAS values, to categorize whether the condition of an individual cancer patient is improving or worsening (versus no change). One advantage of an intra-participant approach is that relative intra-individual changes may be more meaningful for individual patients than absolute changes. However, it is still reasonable to infer that this relative change in VAS scoring is also subjective, as well as being influenced by the factors described above. Observations in other research domains indicate that an intra-individual, VAS-measured improvement in performance-based physical functioning of 20% or more is a minimal clinically important difference that may be used to categorize patients with ankylosing spondylitis as showing or not showing improvement.55
Conclusion
Many patients with head and neck cancer report adverse effects on daily oral function, caused by radiotherapy-induced oral mucositis, that are discordant with objective clinical findings. Especially with low doses of radiation, correlation between patient-reported and clinical manifestations of mucositis is low. In any interventional studies of oral mucositis, patient-reported outcomes should be used to augment clinical observations, as either primary or secondary outcomes, and changes in the values of patient-reported outcomes should be measured on the intra-individual rather than any inter-individual level. If average point or variability estimates at the patient group level are used, subtle but important positive effects on some, but not necessarily all, patients may be masked.
THE AUTHORS
References
- Basch E, Abernethy AP, Mullins CD, Reeve BB, Smith ML, Coons SJ, et al. Recommendations for incorporating patient-reported outcomes into clinical comparative effectiveness research in adult oncology. J Clin Oncol. 2012;30(34):4249-55.
- Reeve BB, Wyrwich KW, Wu AW, Velikova G, Terwee CB, Snyder CF, et al. ISOQOL recommends minimum standards for patient-reported outcome measures used in patient-centered outcomes and comparative effectiveness research. Qual Life Res. 2013;22(8_:1889-1905.
- Macefield RC, Avery KN, Blazeby JM. Integration of clinical and patient-reported outcomes in surgical oncology. Br J Surg. 2013;100(1):28-37.
- Reeve BB, Mitchell SA, Dueck AC, Basch E, Cella D, Reilly CM, et al. Recommended patient-reported core set of symptoms to measure in adult cancer treatment trials. J Natl Cancer Inst. 2014;106(7).
- Chen RC, Chang P, Vetter RJ, Lukka H, Stokes WA, Sanda MG, et al. (2014) Recommended patient-reported core set of symptoms to measure in prostate cancer treatment trials. J Natl Cancer Inst. 106(7).
- Donovan KA, Donovan HS, Cella D, Gaines ME, Penson RT, Plaxe SC, et al. Recommended patient-reported core set of symptoms and quality-of-life domains to measure in ovarian cancer treatment trials. J Natl Cancer Inst. 2014;106(7) pii: dju128. doi: 10.1093/jnci/dju128. Print 2014 Jul..
- Efficace F, Jacobs M, Pusic A, Greimel E, Piciocchi A, Kieffer JM, et al. Patient-reported outcomes in randomised controlled trials of gynaecological cancers: investigating methodological quality and impact on clinical decision-making. Eur J Cancer. 2014;50(11):1925-41.
- Amdal CD, Jacobsen AB, Guren MG, Bjordal K. Patient-reported outcomes evaluating palliative radiotherapy and chemotherapy in patients with oesophageal cancer: a systematic review. Acta Oncol. 2013;52:679-90.
- Chera BS, Eisbruch A, Murphy BA, Ridge JA, Gavin P, Reeve BB, et al. Recommended patient-reported core set of symptoms to measure in head and neck cancer treatment trials. J Natl Cancer Inst. 106(7) pii: dju127. doi: 10.1093/jnci/dju127. Print 2014 Jul.
- Rosenthal DI, Mendoza TR, Chambers MS, Asper JA, Gning I, Kies MS, et al. Measuring head and neck cancer symptom burden: the development and validation of the M. D. Anderson symptom inventory, head and neck module. Head Neck. 2007;29(10):923-31.
- Hebuterne X, Lemarie E, Michallet M, de Montreuil CB, Schneider SM, Goldwasser F. Prevalence of malnutrition and current use of nutrition support in patients with cancer. JPEN J Parenter Enteral Nutr. 2014;38:196-204.
- Vera-Llonch M, Oster G, Hagiwara M, Sonis S. Oral mucositis in patients undergoing radiation treatment for head and neck carcinoma. Cancer. 2006;106:329-36.
- Kushner JA, Lawrence HP, Shoval I, Kiss TL, Devins GM, Lee L, et al. Development and validation of a Patient-Reported Oral Mucositis Symptom (PROMS) scale. J Can Dent Assoc. 2008;74(1):59.
- Siddiqui F, Liu AK, Watkins-Bruner D, Movsas B. Patient-reported outcomes and survivorship in radiation oncology: overcoming the cons. J Clin Oncol. 2014;32:2920-7.
- Dirven L, Taphoorn MJ, Reijneveld JC, Blazeby J, Jacobs M, Pusic A, et al. The level of patient-reported outcome reporting in randomised controlled trials of brain tumour patients: a systematic review. Eur J Cancer. 2014;50: 432-48.
- Macefield RC, Jacobs M, Korfage IJ, Nicklin J, Whistance RN, Brookes ST, et al. Developing core outcomes sets: methods for identifying and including patient-reported outcomes (PROs). Trials. 2014;15:49.
- Johansson B, Brandberg Y, Hellbom M, Persson C, Petersson LM, et al. Health-related quality of life and distress in cancer patients: results from a large randomised study. Br J Cancer. 2008;99(12):1975-83.
- Gussgard AM, Hope AJ, Jokstad A, Tenenbaum H, Wood R. Assessment of cancer therapy-induced oral mucositis using a patient-reported oral mucositis experience questionnaire. PLoS One. 2014;9(3): e91733.
- NCI (2006) NCI , Common Terminology Criteria for Adverse Events v3.0 (CTCAE). NCI.
- Sonis ST, Eilers JP, Epstein JB, LeVeque FG, Liggett WH Jr., Mulagha MT, et al. Validation of a new scoring system for the assessment of clinical trial research of oral mucositis induced by radiation or chemotherapy. Mucositis Study Group. Cancer. 1999;85(10): 2103-13.
- Lockhart PB, Clark J. Pretherapy dental status of patients with malignant conditions of the head and neck. Oral Surg Oral Med Oral Pathol. 1994;77(3):236-41.
- Epstein JB, van der Meij EH, Lunn R, Stevenson-Moore P. Effects of compliance with fluoride gel application on caries and caries risk in patients after radiation therapy for head and neck cancer. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;82(3): 268-75.
- Trotter PB, Norton LA, Loo AS, Munn JI, Voge E, Ah-See KW, et al. Pharmacological and other interventions for head and neck cancer pain: a systematic review. J Oral Maxillofac Res. 2013;3(4):e1.
- Sobin LH, Gospodarowicz MK, Wittekind C, editors. TNM Classification of Malignant Tumours. 7th ed. Wiley-Blackwell; 2009.
- Altman DG. Practical statistics for medical research. London: Chapman & Hall; 1991.
- Sanguineti G, Sormani MP, Marur S, Gunn GB, Rao N, Cianchetti M, et al. Effect of radiotherapy and chemotherapy on the risk of mucositis during intensity-modulated radiation therapy for oropharyngeal cancer. Int J Radiat Oncol Biol Phys. 2012;83(1):235-42.
- Trotti A, Bellm LA, Epstein JB, Frame D, Fuchs HJ, Gwede CK, et al. (2003) Mucositis incidence, severity and associated outcomes in patients with head and neck cancer receiving radiotherapy with or without chemotherapy: a systematic literature review. Radiother Oncol. 2003;66(3):253-62.
- Elting LS, Cooksley CD, Chambers MS, Garden AS. Risk, outcomes, and costs of radiation-induced oral mucositis among patients with head-and-neck malignancies. Int J Radiat Oncol Biol Phys. 2007;68(4):1110-20.
- Elting LS, Keefe DM, Sonis ST, Garden AS, Spijkervet FK, Barasch A, et al. Patient-reported measurements of oral mucositis in head and neck cancer patients treated with radiotherapy with or without chemotherapy: demonstration of increased frequency, severity, resistance to palliation, and impact on quality of life. Cancer. 2008;113(10):2704-13.
- Grossi ML, Goldberg MB, Locker D, Tenenbaum HC. Reduced neuropsychologic measures as predictors of treatment outcome in patients with temporomandibular disorders. J Orofac Pain. 2001;15(4):329-39.
- Denekamp J, Bartelink H, Rubin P. Correction for the use of the SOMA LENT tables. Int J Radiat Oncol Biol Phys. 1996;35(2):417.
- Rugg T, Saunders MI, Dische S. Smoking and mucosal reactions to radiotherapy. Br J Radiol. 1990;63(751):554-6.
- Bjarnason GA, Mackenzie RG, Nabid A, Hodson ID, El-Sayed S, Grimard L, et al. Comparison of toxicity associated with early morning versus late afternoon radiotherapy in patients with head-and-neck cancer: a prospective randomized trial of the National Cancer Institute of Canada Clinical Trials Group (HN3). Int J Radiat Oncol Biol Phys. 2009;73(1):166-72.
- Chen AM, Chen LM, Vaughan A, Sreeraman R, Farwell DG, Luu Q, et al. Tobacco smoking during radiation therapy for head-and-neck cancer is associated with unfavorable outcome. Int J Radiat Oncol Biol Phys. 2009;(2)79:414-9.
- Suresh AV, Varma PP, Sinha S, Deepika S, Raman R, Srinivasan M, et al. Risk-scoring system for predicting mucositis in patients of head and neck cancer receiving concurrent chemoradiotherapy [rssm-hn]. J Cancer Res Ther. 2010;6(4):448-51.
- Sonis ST. Mucositis as a biological process: a new hypothesis for the development of chemotherapy-induced stomatotoxicity. Oral Oncol. 1998;34(1): 39-43.
- Khaw A, Logan R, Keefe D, Bartold M. Radiation-induced oral mucositis and periodontitis - proposal for an inter-relationship. Oral Dis. 2014;20(3): e7-18.
- Borowski B, Benhamou E, Pico JL, Laplanche A, Margainaud JP, Hayat M. Prevention of oral mucositis in patients treated with high-dose chemotherapy and bone marrow transplantation: a randomised controlled trial comparing two protocols of dental care. Eur J Cancer B Oral Oncol. 1994;30B(2):93-7.
- Yeoh A, Gibson R, Yeoh E, Bowen J, Stringer A, Giam K, et al. Radiation therapy-induced mucositis: relationships between fractionated radiation, NF-kappaB, COX-1, and COX-2. Cancer Treat Rev. 2006;32(8):645-51.
- Vatca M, Lucas JT Jr., Laudadio J, D'Agostino RB, Waltonen JD, Sullivan CA, et al. Retrospective analysis of the impact of HPV status and smoking on mucositis in patients with oropharyngeal squamous cell carcinoma treated with concurrent chemotherapy and radiotherapy. Oral Oncol. 2014;50(9): 869-76.
- Allison PJ, Guichard C, Gilain L. A prospective investigation of dispositional optimism as a predictor of health-related quality of life in head and neck cancer patients. Qual Life Res. 2000;9(8):951-60.
- Reich M, Leemans CR, Vermorken JB, Bernier J, Licitra L, Parmar S, et al. Best practices in the management of the psycho-oncologic aspects of head and neck cancer patients: recommendations from the European Head and Neck Cancer Society Make Sense Campaign. Ann Oncol. 2014;25(11):2115-24.
- Steingrímsdóttir OA, Vøllestad NK, Røe C, Knardahl S. Variation in reporting of pain and other subjective health complaints in a working population and limitations of single sample measurements. Pain. 2004;110(1-2):130-9.
- Von Korff M, Saunders K. The course of back pain in primary care. Spine (Phila Pa 1976). 21:2833-7; discussion 2838-9.
- Ohrn KE, Wahlin YB, Sjoden PO. Oral status during radiotherapy and chemotherapy: a descriptive study of patient experiences and the occurrence of oral complications. Support Care Cancer. 2001;9(4):247-57.
- Goldstein NE, Genden E, Morrison RS. Palliative care for patients with head and neck cancer: "I would like a quick return to a normal lifestyle". JAMA. 2008;299(15):1818-25.
- Hammerlid E, Ahlner-Elmqvist M, Bjordal K, Biorklund A, Evensen J, Boysen M, et al. A prospective multicentre study in Sweden and Norway of mental distress and psychiatric morbidity in head and neck cancer patients. Br J Cancer. 1999;80(5-6):766-74.
- Dodd MJ, Dibble S, Miaskowski C, Paul S, Cho M, MacPhail L, et al. A comparison of the affective state and quality of life of chemotherapy patients who do and do not develop chemotherapy-induced oral mucositis. J Pain Symptom Manage. 2001;21(6):498-505.
- Tan JD, Butow PN, Boyle FM, Saw RP, O'Reilly AJ. A qualitative assessment of psychosocial impact, coping and adjustment in high-risk melanoma patients and caregivers. Melanoma Res. 2014;24(3):252-60.
- Horney DJ, Smith HE, McGurk M, Weinman J, Herold J, Altman K, et al. Associations between quality of life, coping styles, optimism, and anxiety and depression in pretreatment patients with head and neck cancer. Head Neck. 2011;33(1):65-71.
- Kwok W, Bhuvanakrishna T. The relationship between ethnicity and the pain experience of cancer patients: a systematic review. Indian J Palliat Care. 2014;20(3):194-200.
- Morton RP. Studies in the quality of life of head and neck cancer patients: results of a two-year longitudinal study and a comparative cross-sectional cross-cultural survey. Laryngoscope. 2003;113(7):1091-103.
- Rahim-Williams B, Riley JL 3rd, Williams AK, Fillingim RB A quantitative review of ethnic group differences in experimental pain response: do biology, psychology, and culture matter? Pain Med. 2012;13(4):522-40.
- Allison PJ, Locker D, Feine JS. Quality of life: A dynamic construct. Soc Sci Med. 1997;45(2):221-30.
- van Weely SF, van Denderen JC, Steultjens MP, Nurmohamed MT, Dijkmans BA, Dekker J, et al. (2013) What do we miss? ASAS non-responders on anti-TNF therapy show improvement in performance-based physical function. Rheumatology (Oxford). 2013;52(10):1884-9.





