Abstract
Objectives: Oral hairy leukoplakia (OHL) is caused by Epstein-Barr virus (EBV) and is often associated with HIV and other immunosuppressive conditions. It is rare in HIV-negative patients, but has been reported in patients who use immune-modulating medications (e.g., cyclosporine). The objectives of this study were to determine the occurrence of OHL in HIV-negative patients and report Langerhans cell counts in these lesions.
Study design: A series of 7 new cases of OHL among HIV-negative patients is described. Langerhans cells were counted using an immunoperoxidase stain for CD1a and light microscopy.
Results: The 7 patients were male, ranging in age from 26 to 69 years. Clinically, all lesions were diagnosed as leukoplakia on the lateral border of the tongue. Microscopic examination revealed hyperparakeratosis and candidiasis in some cases, acanthosis and a band-like zone with clearing of cells in the upper spinous layer, which were EBV-positive by in-situ hybridization. There was a significant decrease in Langerhans cell counts in OHL patients.
Conclusion: OHL can occur in HIV-negative patients.
First described in 1984, oral hairy leukoplakia (OHL) is a mucosal disease caused by infection with Epstein-Barr virus (EBV).1 OHL is a benign lesion, presenting most often as an opportunistic infection in immune-suppressed patients.2,3 OHL is the most common EBV-related lesion in patients with AIDS, and its presence in HIV-infected people is a sign of severe immunosuppression.3 It has also been described in patients who are immunosuppressed for reasons other than HIV infection.4 Only a few reports exist of OHL occurring in patients who are apparently immune competent.4-10
EBV infection of epithelial cells in OHL was first described by Greenspan et al. in 198511; it is now well established that EBV is the cause of OHL. Primary infection occurs in the tonsillar area, followed by latent infection of resting B-cells, with probable later reactivation and reinfection of epithelial cells.12
Clinically, OHL manifests as unilateral or bilateral, white or grey patches on the lateral margins, dorsal or ventral surfaces of the tongue that cannot be wiped off (Fig. 1). It exhibits a range of appearances from faintly visible white streaks to deep furrowed, “shaggy” surfaced leukoplakia.13 It also occurs occasionally at other oral and oropharyngeal sites.13-15
Microscopically, OHL features hyperparakeratosis with an irregular corrugated surface; a zone of cells showing clearing of cytoplasm and peripheral nuclear beading of chromatin in the superficial stratum spinosum; and acanthosis.13 Nuclear beading and margination of chromatin is caused by EBV replication.13 Candidiasis is often present.13 Because the presence and etiologic role of EBV nucleic acids in epithelial cells has been well documented, in-situ hybridization is a valuable tool for confirming the diagnosis of OHL and for distinguishing it from other white lesions, such as hyperkeratosis, with similar clinical and histologic features.11,16,17
OHL has been described as a clinical indicator of immunosuppression.3 Its association with HIV, AIDS, transplant recipients and other patients on immunosuppressive medication, as well as hematologic malignancies, has now been extensively documented.13,18 Although, initially, all cases of OHL in HIV-positive patients were in men who have sex with men, it has now been seen in all groups infected with HIV.13
A large number of cases of OHL have been reported in patients who were immunodeficient for reasons other than HIV infection, such as transplant recipients using immunosuppressive medication, people with asthma using steroid inhalers and patients with primary blood disorders.3,4,8,19 OHL has also been reported in conditions with an underlying immune dysfunction, including autoimmunity or hypersensitivity.4,20-23 A comprehensive list of HIV-negative patients with OHL has recently been published.4
Notably, OHL can occur in HIV-negative patients who are not taking immunosuppressive medication or therapy of any form, who are apparently immune competent and who have no apparent predisposition. Although only 8 such cases could be traced in the literature from 1989 to 2015,4,5,7-10 from this body of literature, it is clear that OHL is not limited to HIV-positive or immune-suppressed patients.
In this paper, we present a series of 7 new cases of OHL in patients who were not evidently immune suppressed. At the time of presentation, the HIV status of these patients was not known, and they were tested after the diagnosis of OHL was made. Because Langerhans cell numbers have been reported to be lower in OHL patients with AIDs,24,25 we also aimed to determine whether Langerhans cell counts in OHL patients who were HIV negative were different from counts in “normal” control tissues.24 The purpose of this paper is to highlight to dentists that OHL can occur in patients who are not immunosuppressed.
Materials and Methods
Six paraffin-embedded formalin-fixed specimens were retrieved from the archives of the pathology department at the University of Western Ontario and 1 from the College of Dentistry, University of Saskatchewan, for review of the microscopic features and clinical reports. Medical histories were obtained from the pathology requisition forms and the submitting dentist. All specimens were stained with hematoxylin and eosin and periodic acid–Schiff stain (with and without diastase digestion) to determine the presence or absence of Candida albicans and glycogen.
The study cases were compared with non-inflamed tongue control cases where only hyperparakeratosis presenting as a leukoplakia was present (normal controls) and HIV-positive, OHL-negative patients who presented with a leukoplakia of the lateral tongue.
Epstein-Barr virus in-situ hybridization
All tissues were examined for the presence of EBV by means of in-situ hybridization using a standard laboratory protocol (EBV (EBER) PNA Probe/Fluorescein, Code Y5200, and PNA ISH Detection Kit, Code K5201, Dako, Mississauga, Ontario, Canada).
Langerhans cells
The number of Langerhans cells (antigen-presenting cells) was evaluated using a routine immunoperoxidase stain for CD1a (monoclonal mouse anti-human, clone 010, Code IR069, Dako, Mississauga, Ontario, Canada). CD1a-positive cells were counted in non-overlapping, microscopic fields at 200× original magnification and recorded per unit area (mm2) of the squamous epithelium of lesional tissue (n = 7), normal control tissue (n = 7) and HIV-positive controls (n = 9). For each case, the entire area of epithelium present was measured, and the average number of Langerhans cells per mm2 was calculated. Cell counts were made by 3 investigators blinded to the histopathologic diagnosis.
Statistical analysis
A 2-tailed unpaired Student t test with Welch correction was conducted on the Langerhans cell counts, using GraphPad Prism 7 (GraphPad Software, La Jolla, Calif.). Statistical significance was set at 0.05.
Ethics approval
We have read the Helsinki Declaration and have followed the guidelines in this investigation. Approval was obtained from Western University Health Science Research and Ethics Board (HSREB number 105016).
Results
All 7 patients were male, median age 51 years (range 26–69 years), and all lesions presented as a white patch on the tongue, all of which healed uneventfully. A summary of the clinical findings is presented in Table 1. All patients either had a smoking history or other medical conditions, such as diabetes, hypertension, hyperlipidemia and gout (Table 1). Patients were receiving medications for these conditions, all of which were controlled, but duration and dosage were not disclosed.
All patients showed similar histopathologic findings (Fig. 2A), were HIV negative and were positive for EBV detected by in-situ hybridization (Fig. 2C). After diagnosis of OHL, patients were tested for HIV. Because OHL is regarded as pathognomonic for immunodeficiency status,3 in particular for HIV infection, is an AIDS-associated lesion,11 may be an initial indicator of HIV infection13 and also for logistical reasons, as the patients were seen at different offices in areas around the province, a second test for HIV was not completed. In all cases, the clinician reported that the lesions resolved without recrudescence or recurrence and without any additional treatment.
Langerhans cell counts
The median Langerhans cell count per microscopic field (at 200x original magnification) in OHL, normal control patients and HIV-positive controls was 2 (range 0–36), 6 (range 0–95) and 11 (range 0–61) respectively, considered highly significant (p < 0.001) (Table 2). Immunohistochemistry for CD1a to identify Langerhans cells is shown in Figures 2E and 2F.
Figure 1: Oral hairy leukoplakia of the left lateral border of the tongue, presenting as a white lesion with an irregular, corrugated and “shaggy” surface.
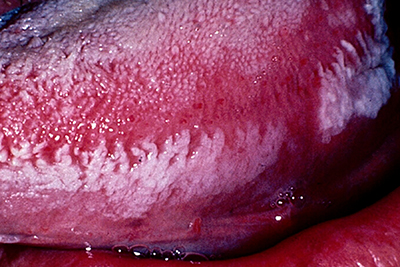
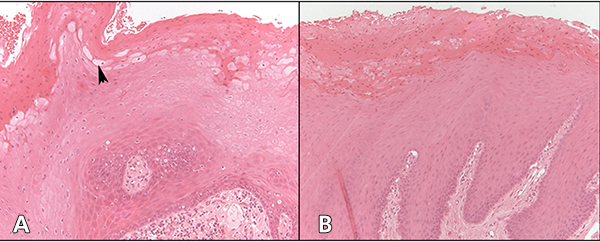
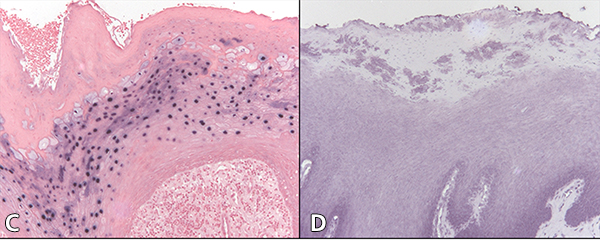
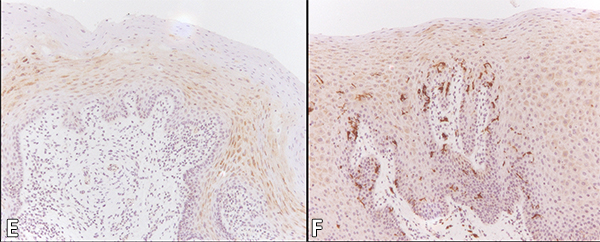 Figure 2: Cytology of oral hairy leukoplakia (OHL) in patient 4 (findings were similar in all 7 cases) in A, C and E, compared with control tissue in B, D and F (original magnification ×100). A: Hematoxylin and eosin stain shows hyperparakeratinized stratified squamous epithelium with surface corrugation, acanthosis and clearing of cells (“balloon cells,” arrowhead) in the upper stratum spinosum. B: Control tissue shows hyperkeratosis and acanthosis, but no significant ballooning of cells, nuclear margination or beading of chromatin. C: In OHL patient, in-situ hybridization for Epstein-Barr virus (EBV) is positive in the nuclei of epithelial cells. D: Control tissue is negative for EBV RNA. E: OHL is negative for CD1a in this section; no Langerhans cells are present. F: In control tissue, dark brown immunohistochemical staining for CD1a reveals more numerous Langerhans cells than in OHL cases.
Figure 2: Cytology of oral hairy leukoplakia (OHL) in patient 4 (findings were similar in all 7 cases) in A, C and E, compared with control tissue in B, D and F (original magnification ×100). A: Hematoxylin and eosin stain shows hyperparakeratinized stratified squamous epithelium with surface corrugation, acanthosis and clearing of cells (“balloon cells,” arrowhead) in the upper stratum spinosum. B: Control tissue shows hyperkeratosis and acanthosis, but no significant ballooning of cells, nuclear margination or beading of chromatin. C: In OHL patient, in-situ hybridization for Epstein-Barr virus (EBV) is positive in the nuclei of epithelial cells. D: Control tissue is negative for EBV RNA. E: OHL is negative for CD1a in this section; no Langerhans cells are present. F: In control tissue, dark brown immunohistochemical staining for CD1a reveals more numerous Langerhans cells than in OHL cases.
| Patient | Site | Clinical description |
Medical conditions |
Smoking history |
Biopsy type | Candida | Follow-up without recrudescence/recurrence |
|---|---|---|---|---|---|---|---|
| 1 | Left ventral tongue | Leukoplakia | None | Current smoker | Not specified | + | 12 years |
| 2 | Right lateral/ ventral tongue | Leukoplakia | None | Previous smoker (quit 40 years previously) | Incisional | − | 5 years |
| 3 | Right lateral tongue | White patch | None | Current smoker | Not specified | + | 4 years |
| 4 | Left tongue | Leukoplakia | Osteoarthritis | No history | Not specified | − | 4 years |
| 5 | Right lateral/ ventral tongue | Leukoplakia | Diabetes type II, hypertension, hyperlipidemia | No history | Not specified | − | 1.5 years |
| 6 | Left lateral tongue | White patch | Diabetes type II, hypertension | No history | Incisional | + | 7 months |
| 7 | Right lateral tongue | 2 white lesions | Diabetes type II, hypertension, gout | No history | Not specified | − | Lost to follow-up |
| Patient LHC Counts (n=7) | Normal Control LHC Counts (n=7) | HIV Positive Control LHC Counts (n=9) | ||||
|---|---|---|---|---|---|---|
| Total number of LHC | Mean number of LHC/ mm2 | Total number of LHC | Mean number of LHC/ mm2 | Total number of LHC | Mean number of LHC/ mm2 | |
| 78 | 6.5 | 55 | 3.9 | 149 | 9.9 | |
| 7 | 0.5 | 0 | 0 | 240 | 13.3 | |
| 8 | 0.9 | 196 | 7.5 | 25 | 3.1 | |
| 36 | 1.7 | 42 | 4.7 | 229 | 16.4 | |
| 31 | 1.9 | 200 | 8.3 | 393 | 49.1 | |
| 247 | 10.3 | 56 | 2.4 | 280 | 23.3 | |
| 21 | 1.8 | 648 | 38.1 | 196 | 11.5 | |
| 320 | 21.3 | |||||
| 42 | 3.5 | |||||
| Total Counts and Overall Mean | 428 | 4 | 1197 | 9.7 | 1874 | 15.7 |
| Median number of LHC/mm2 | 2 | 6 | 11 | |||
Discussion
Few cases of OHL have been reported in immune competent patients.4 Four patients in this case series had pre-existing medical conditions, which were controlled. For 3 of the patients reported here, only possible tobacco use and perhaps other unknown factors inducing local immunosuppression could be considered to play a role as so-called “cryptic immunopression.”13 There have been reports of OHL cases not associated with immunosuppression,5,26 but it was not clear whether local immunosuppression was absolutely ruled out. The possibility of cryptic immunosuppression as suggested by Greenspan et al.13 must be considered and perhaps more thoroughly investigated in these patients. Such investigation would include whether patients are using inhaled steroids, immune-modulating drugs,27 such as cyclosporine or azathioprine, are diabetic or have some factor creating a local immunosuppression. Chambers et al.4 suggest that “systemic conditions leading to deficient immune system function and local factors together facilitate the development of OHL.”
EBV occurs worldwide, and as many as 90–95% of adults in the United States between the ages of 35–40 years have been infected. Transmission of EBV usually occurs through intimate contact with the saliva of an infected person and is not normally transmitted through air or blood. After the initial infection has been resolved, the virus can remain latent in B cells and can cause a lytic infection in the oropharynx. Recrudescence of the viral infection is caused by immunosuppression and reactivation of the virus from its latent state.12,14 The mechanism by which EBV causes OHL has not yet been fully determined, although several possibilities exist: infection by virus present in saliva; reactivation of latent virus in tongue epithelial cells; or transmission of EBV from lymphocytes to epithelial cells during periods of relative or active immunosuppression.12,28
Three of the patients reported here had type II diabetes, which was controlled in all cases. When diabetes is not controlled, chronic immunosuppression, delayed healing and/or salivary hypofunction may result in oral mucosal disease.29 At least two studies have found increased EBV DNA and OHL in people with diabetes.4,30 Milagres et al.30 concluded that EBV infection of the lateral border of the tongue of people with minor immunodeficiency (e.g., pregnant women and people with diabetes) was considerably higher than in healthy people.
Another factor contributing to the development of OHL may be the long-term use of steroid medications. In a recent study, Chambers et al.4 concluded that 28 patients who were using inhaled steroids for asthma or chronic obstructive pulmonary disease were at risk of developing OHL.4 Rushing et al.19 reported the occurrence of OHL in 2 patients who were on immunosuppressive therapy, concluding that it probably resulted from new or persistent infection with EBV. The mechanism appears to be a local immunosuppressive effect.4,31
The development of OHL was previously seen to arise only in patients with immunodeficiency. In a recent study, Chambers et al.4 recorded OHL lesions in some patients with normal complete blood counts who were not taking immunosuppressive medications. Although these patients had no known risk factors for OHL and were presumed to be HIV negative, they had not been tested for HIV. In these patients, other factors were considered to be contributing to the development of OHL, with 1 case concluded to be an idiopathic transitory disorder following the lesion’s spontaneous regression.4
In HIV-positive OHL patients, the number of Langerhans cells in the epithelium is generally decreased.24 Walling et al.24 suggest that EBV replication is directly responsible for decreased Langerhans cell counts in the oral epithelium in cases of OHL. In our HIV-negative patients, the number of Langerhans cells was decreased in those with OHL lesions compared with control patients. Walling et al.24 found that EBV replication decreases oral Langerhans cell counts independent of HIV infection, which is consistent with our findings in HIV-negative patients. Our patient 6 had a higher Langerhans cell count in an area of epithelium where in-situ hybridization was negative for EBV, but clearly lower in the area of epithelium where EBV was present. In this case, Candida hyphae were also present.
Generally, OHL does not require any treatment, as it tends to resolve spontaneously,4,21 as in the patients described here, whose OHL resolved after biopsy. However, recurrence is frequent in patients who are immune suppressed.13
A variety of treatment options for lesions that do not resolve have been examined or suggested in the literature. For example, podophyllin, a cytostatic plant toxin, has been suggested, although some adverse effects have been reported.32,33 A combination of 25% podophyllin and 5% acyclovir cream has been reported to be effective.34 A single case report has recommended topical gentian violet.35 Antiviral therapy may be most useful to treat OHL, although there is a lack of consensus on the most efficacious treatment regimen. Resolution has been found to occur in most cases treated with oral acyclovir (800 mg every 6 h for 20 days), valacyclovir (1000 mg orally every 8 h for 28 days) and desciclovir (250 mg every 8 h for 14 days).36,37 Medical treatment may be particularly useful in patients who are not immune suppressed and who have persistent, recurrent or recrudescent lesions.13
In this paper, we describe 7 cases of OHL in patients who were HIV negative and not taking any immunosuppressive medication. It is important that dentists be aware that OHL can develop in those who are not immune deficient and perhaps have “cryptic” immunosuppression (e.g., local immunosuppression resulting from inhaled steroids), as suggested by Greenspan et al.13 This is important, as OHL generally develops in a site that is at high risk for epithelial dysplasia and oral squamous cell carcinoma, making accurate diagnosis essential to avoid overtreatment.





