Abstract
Objectives: To summarize evidence relating cannabis smoking and oral disease and highlight any potential influence of cannabis smoking on clinical care and dental public health.
Methods: Using rapid evidence review, a librarian facilitated a systematic search of 5 electronic databases in August and September 2018 and updated it in March 2019, yielding 581 publications. Two researchers screened the documents using pre-established inclusion criteria: article was based on primary or secondary data; cannabis smoking was an exposure; at least 1 cannabis-related oral health outcome was reported; participants were humans; and the article was available in English or French. Data from retained articles were analyzed for themes without meta-analysis.
Results: We synthesized and summarized 23 articles in 2 broad categories: cannabis and oral disease; and cannabis, clinical care and dental public health. Current evidence shows that smoking cannabis is harmful to the health of the periodontium. The association between smoking cannabis and other oral disease (dental caries, soft tissue lesions and oral cancers) is sparse and inconsistent, although studies suggest that cannabis smoking is an underlying risk factor. Cannabis smoking can lead to an altered mental state that can delay dental treatment of the patient. Further, interactions between smoked cannabis and adrenaline-containing local anesthetics can result in life-threatening consequences.
Conclusions: Cannabis smoking is harmful to the periodontium. Further research is needed to fully understand how cannabis smoking affects oral disease and how dental professionals should integrate this knowledge into clinical care and dental public health.
Cannabis is the most abused drug in most countries of the world.1 An estimated 147 million people use cannabis globally, mainly for recreational purposes.2 In Canada, 4.2 million people aged 15 and older reported using cannabis products in the previous 3 months, and a third of surveyed youths had consumed cannabis at least once by their 15th birthday.3 In addition, national consumption levels have risen annually since 1985, driven mainly by cannabis use among Canadians aged 25 years and older.4
Smoking cannabis has adverse effects on multiple human organs including the oral cavity.5 The plant comprises over 400 chemical entities with the two main compounds, delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD), shown to have opposing effects on many human organs. People who smoke cannabis usually experience an altered mental state (psychoactive feeling) that commences within a few minutes and can last up to 3 h.6 The long-term effects of cannabis use include low birthweight, structural, functional and chemical changes in the brain, early onset psychosis, strokes, testicular cancer, suicide tendencies and deficiency in motor function and learning.5 It is estimated that up to 17% of people who start consuming cannabis as adolescents will develop cannabis use syndrome (irritability, anger, depression, difficulty sleeping, craving and decreased appetite).7 In the oral cavity, smoking cannabis has been associated with periodontal disease,8 dental caries9 and oral cancers.10
The oral health effects of cannabis smoking are likely to increase in Canada following the legalization of the substance. Under the Cannabis Act, Canadians aged 18 years and older can legally possess and share up to 30 g of dried cannabis or its equivalent in authorized public spaces. People can also grow up to 4 plants for their own use.11 Most cannabis users in Canada (78%) prefer to smoke the dried flowers or leaves of the plant compared with eating and vaping.11 We can expect increased exposure of the oral tissues to cannabis smoke as more Canadians consume the substance. In addition, cannabis users can now discuss their oral health concerns with their dental professionals and expect informed responses. Dental professionals can expect to deal increasingly with clients who present with cannabis-related pathologies or concerns.
However, literature to help dental professionals offer evidence-based treatment and advice to their clients is limited. In fact, we did not find a single Canadian study on cannabis and oral health. The purpose of this rapid evidence review is to synthesize and summarize evidence on cannabis smoking and oral health that can inform the actions of dental professionals and begin to fill this important knowledge gap. The specific objectives were to summarize evidence on a relation between cannabis smoking and periodontal disease (dental caries, oral soft tissue lesions and conditions and oral cancers); and to describe the effect of cannabis smoking on clinical dental care and dental public health.
Methods
We adopted the “knowledge to action evidence summary” method, which is appropriate for synthesizing evidence to inform decisions in a health care setting.12 This systematic method includes a needs assessment and research question, a systematic literature search, screening and selection of studies, data extraction and narrative synthesis.12
Needs Assessment and Research Question
The question that guided the review was: what is the evidence linking cannabis smoking, oral disease and oral health care? After reflection, our research team concluded that this would be the most significant question on the minds of dental professionals and the Canadian oral health community, following the recent legalization of cannabis. The expertise of the team cut across clinical dentistry, epidemiology, medical anthropology, substance abuse and pain management.
Systematic Literature Search
A faculty of dentistry librarian facilitated a comprehensive and systematic search of relevant literature in August and September 2018 and updated the results in March 2019. Key words and MeSH terms were combined to search the Medline Ovid database, and the search strategy was then translated to 4 additional electronic databases: Scopus, Global Health, CINHAL and Embase. No date limits were applied to the search to ensure that it was comprehensive and did not miss any relevant articles. All 581 publications from the 5 electronic databases were transferred to Rayyan software13 for screening and selection. Figure 1 shows details of the search strategy.
- Cannabis
- (cannabis or cannabino* or cannabis).tw,kf.
- 1 or 2
- exp Oral Health/
- exp Oral Hygiene/
- stomatognathic diseases/ or exp mouth diseases/ or exp pharyngeal diseases/ or exp tooth diseases/
- ((dental* or oral* or gingiv* or tooth or teeth or endodont* or periodont*) adj3 (disease? or health* or hygiene)).mp.
- ((mouth or gingiv* or tongue) adj3 (cancer* or tumo?r? or neoplas*)).tw,kf.
- ((tooth or teeth) adj3 loss).tw,kf.
- or/4–9
- 3 and 10
Figure 1: Details of the search strategy.
Screening and Selection of Studies
To be included, an article had to (i) provide primary or secondary data on cannabis use and oral health, (ii) include cannabis smoking as an exposure or risk factor, (iii) report at least 1 cannabis-related oral health outcome, (iv) look at human participants and (v) be available in English or French. We excluded non-systematic reviews (e.g., summaries, commentaries) and anonymous publications. Using Rayyan software,13 2 researchers (MK, NE), independently screened the titles and/or abstracts of the articles and assigned each to 1 of 3 groups: included, undecided or excluded. The researchers then jointly reviewed the abstracts in the included and undecided groups and agreed on abstracts to undergo full text screening. In the next step, full texts of 48 articles were obtained and screened in a similar procedure. This resulted in 23 articles that met all criteria (Fig. 2).
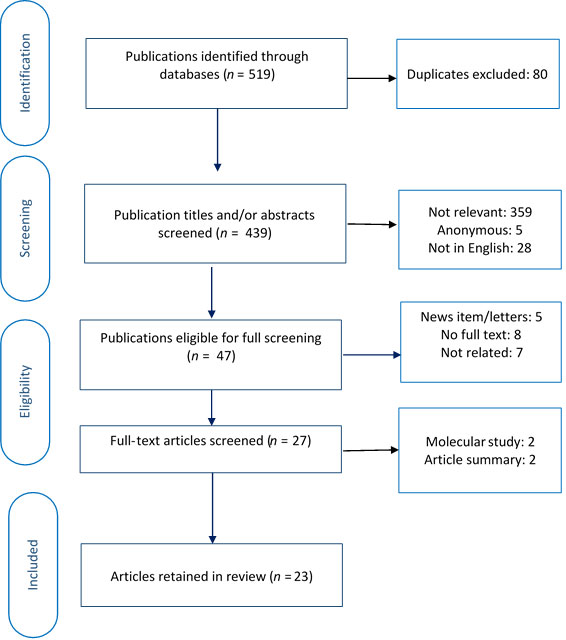
Figure 2:PRISMA diagram showing selection of articles for rapid evidence review.
Data Extraction and Narrative Synthesis
Relevant data were extracted from the 23 articles and transferred to a spreadsheet; this information included the purpose and objectives of the study, the design, study population, sample size, outcomes or results, key messages and conclusion. The data were then pooled for a thematic analysis and a narrative synthesis.14 The quantitative data were linked with the specific study when examples were provided.
Results
The 23 articles included in this evidence review encompasses a variety of study designs: 6 cross-sectional surveys,15-19,24 7 case/clinical reports,28,30-35 5 case–control studies10,20-23 3 cohort studies,8,9,25 1 clinical trial26 and 1 systematic review and meta-analysis.27 The sample size of the reviewed studies varied from 1 participant in a case report28 to 9163 participants in a survey.17 We present the synthesized evidence under 2 categories with 6 sub-themes: cannabis smoking and oral disease (cannabis and periodontal disease, cannabis and caries, cannabis and oral soft tissues, cannabis and oral cancers) and cannabis smoking, clinical care and population oral health (cannabis and clinical care, cannabis and dental public health). A summary of the results is found in Table 1. The table also indicates the level of evidence for each retained article, based on its study design.29 The strength of the evidence ranges from level I to level IV.
|
Article |
Purpose/aims/objectives |
Study design/participants/location/ |
Summary of key |
|---|---|---|---|
| aOR = adjusted odds ratio, CI = confidence interval, DNA = deoxyribonucleic acid, PR = prevalence ratio, OR = odds ratio, RNA = ribonucleic acid, THC = tetrahydrocannabinol. | |||
| Cannabis and periodontal disease | |||
| Chisini et al.27 | To analyze whether use of cannabis is associated with periodontitis. | Systematic review and meta-analysis Level I evidence | A positive association exists between the use of cannabis and periodontitis (PR 1.12, 95% CI 1.06–1.19) |
| Jamieson et al.24Jamieson et al.24 | To investigate the effects of tobacco, cannabis, alcohol and petrol sniffing on periodontal disease among young Australian Aboriginal adults.To investigate the effects of tobacco, cannabis, alcohol and petrol sniffing on periodontal disease among young Australian Aboriginal adults. | Nested cross-sectional study in long-standing prospective study; participants (mean age 18 years) from Aboriginal communities in Australia’s Northern Territory (n = 425) Level II evidence | A 1.5-fold increase in periodontal disease among tobacco users was no longer significant after adjusting for confounders. |
| Lopez and Baelum17 | To investigate the association between cannabis use and destructive periodontal disease among adolescents. | Survey; high school students aged 12–21 years in Chile (n = 9163) Level IV evidence | There was no significant association between clinical attachment loss (≥ 3 mm) and cannabis use. |
| Meier et al.8 | To test associations between cannabis use over 20 years and a variety of physical health indexes at early midlife. | Birth cohort; periodontal health of participants in the Dunedin study, evaluated at 26 and 38 years (n = 1037) Level II evidence | Cannabis use for up to 20 years was associated with periodontal disease. |
| Momen-Heravi and Kang31 | To describe the presentation and treatment of periodontitis in a young woman with a history of chronic cannabis use. | Clinical report; 23-year old female in a dental clinic in the USA (n = 1) Level IV evidence | Localized papillary and marginal gingival enlargement was found in the anterior mandible. |
| Rawal et al.30 | To describe the clinical status of 2 patients with cannabis-related gingival enlargement and review the literature on oral complications of cannabis use. | Case report; 2 patients aged 23 and 42 years in the USA (n = 2) Level IV evidence | Chronic cannabis use may cause phenytoin-like gingival enlargement. |
| Shariff et al.19 | To examine the relation between frequent recreational cannabis use and the prevalence of periodontitis. | Participants in 2011–2012 National Health and Nutrition Examination Survey (USA) with mean age 44.5 years (n = 1938) Level IV evidence | The sites and severity of periodontal disease was significantly higher among frequent recreational cannabis users than among non-users. |
| Thomson et al.25 | To determine whether cannabis smoking is a risk factor for periodontal disease. | Birth cohort; data from participants aged 32 years from the Dunedin study, New Zealand (n = 903) Level II evidence | People with greater exposure to cannabis had significantly higher levels of periodontal disease compared with non-smokers after controlling for potential confounders. |
| Cannabis and dental caries | |||
| Ditmyer et al.9 | To determine trends in tobacco-cannabis use in Nevada adolescents and the effect on dental health status. | Retrospective cohort study; dental health screening of adolescents aged 13–18 years in Nevada, USA, collected over 8 years (n = 66 941 screenings) Level II evidence | The percent mean difference (PMD) in DMFT among cannabis smokers varied from 44.94% in year 1, increased to 51.74% and dropped to 19.76% in year 3. |
| Schulz-Katterbach et al.23 | To investigate the hypothesis that regular cannabis use increases the risk of caries. | Case–control study with 42 cases, 43 controls, aged 18–25 years in Switzerland (n = 85) Level III evidence | Cannabis users had significantly higher decayed surface values compared with tobacco smokers, although there was no significant difference in decayed and filled surfaces values. |
| Cannabis and oral soft tissues | |||
| Darling and Arendorf21 | To determine the effects of cannabis smoking on the oral soft tissues. | Case–control study; 300 cannabis/tobacco/methaqualone cases, 152 tobacco smokers, 189 non-smokers in Cape Town, South Africa (n = 641) Level III evidence | Prevalent lesions and conditions included: xerostomia (transient), leukoedema, nicotinic stomatitis, erythroplakia, denture stomatitis, leukoplakia and Fordyce’s granules. |
| Darling et al.20 | To determine the effects of cannabis smoking on oral candidiasis as well as the oral prevalence and density of Candida albicans. | Case–control study of 55 cannabis smokers and 58 non-smokers in Cape Town, South Africa (n = 108) Level III evidence | Cannabis smoking increases the intraoral prevalence of C. albicans. |
| Rawal et al.30 | As above | As above | As above |
| Cannabis and head and neck cancers | |||
| Ahrens and Bressi18 | To investigate the relation between drug smoking and oral cancer. | Evaluation of 18-45 year olds (mean age, 29 years) in University Hospital Cheiti, Italy (n = 178) Level IV evidence | Smoking cannabis is a potential risk factor for oral cancer. |
| Almadori et al.33 | To describe a case of carcinoma of the tongue in a male 23-year-old “regular” cannabis smoker. | Case report; 23-year patient with history of cannabis smoking, in Rome, Italy (n = 1) Level IV evidence | Cannabis and tobacco smoke may have a direct carcinogenic effect. |
| Caplan and Brigham34 | To describe squamous cell carcinoma of the tongue in 2 chronic cannabis users in the absence of other risk factors. | Case reports; 2 male Australians, aged 37 and 52 years (n = 2) Level IV evidence | Smoking cannabis is a potential risk factor for upper respiratory track cancers. |
| Donald35 | To investigate the association between cannabis smoking and head and neck cancers among young persons. | Case reports; patients aged 19–38 years (mean 28.5 years) in USA (n = 11) Level IV evidence | Smoking cannabis is suspected as a risk factor for squamous cell carcinoma of the head and neck region. |
| Donald32 | To describe the clinical presentation of 6 young regular cannabis users with advanced head and neck cancer. | Case reports; patients aged 19, 20, 27, 28, 34 and 38 years in California, USA (n = 6) Level IV evidence | Delta-9 THC is a potential carcinogen and may alter DNA, RNA, and protein synthesis. |
| Marks et al.10 | To investigate the association of cannabis use with risk of oropharyngeal and oral tongue cancers. | Meta-analysis; pooled data from 9 case–control studies: 1921 oropharyngeal cases, 356 oral tongue cases and 7639 controls in the USA and Latin America (n = 9916) Level III evidence | Cannabis smokers had an elevated risk of oropharyngeal (aOR 1.24, 95% CI 1.06–1.47) and a reduced risk of oral tongue cancer (aOR 0.47, 95% CI 0.29– 0.75), compared with non-smokers. |
| Rosenblatt et al.22 | To determine the association of cannabis use with oral squamous cell carcinoma. | Case–control study; 407 cases and 615 controls, in Washington, USA (n = 1022) Level III evidence | Cannabis use was not associated with oral squamous cell carcinoma (aOR 0.9, 95% CI 0.6–1.3). |
| Cannabis use and clinical care | |||
| Ditmyer et al.9 | As above | As above | As above |
| Grafton et al.28 | To illustrate the need for cannabis-related education for oral health professionals. | Case report; 27-year old patient in USA (n = 1) Level IV evidence | Dentists should be equipped to identify and manage possible effects of cannabis use in the dental clinic. |
| Gregg et al.26 | To determine the cardiovascular effects of cannabinols. | Clinical trials; healthy male patients aged 19–28 years in Carolina, USA (n = 2) Level I evidence | Smoking cannabis 72 h before general anesthesia led to sustained postoperative tachycardia. There was no advantage to using THC over diazepam or placebo as pre-medication. |
| Momen-Heravi Heravi and Kang31 | As above | As above | Proposed treatment for patient with cannabis-related periodontitis to combine behaviour change counselling with surgical and non-surgical procedures. |
| Cannabis and dental public health | |||
| D’Amore et al.15 | To examine the effects of alcohol, stimulants, opioids and cannabis on oral health. | Substance-dependent individuals, with mean age 38 years in Boston, USA (n = 536) Level IV evidence | Over half (57.8%) of cannabis users reported poor oral health, although there was no significant association between cannabis use and oral health status. |
| Ditmyer et al.9 | As above | As above | As above |
| Meier et al.8 | As above | As above | As above |
| Rosenblatt et al.22 | As above | As above | As above |
| Schulz-Katterbach et al.23 | As above | As above | As above |
| Shariff et al.19 | As above | As above | As above |
| Siziya et al.16 | To explore associations between sociodemographic variables and self-reported oral hygiene. | Survey; adolescents aged 13–15 years in Zambia (n = 2257) Level IV evidence | Respondents who smoked cannabis were more likely to report poor oral hygiene (aOR = 1.04, 95% CI 1.02–1.07). |
| Thomson et al.25 | As above | As above | As above |
Cannabis Smoking and Oral Disease
Cannabis Smoking and Periodontal Disease
The literature suggests that frequent and long-term smoking of cannabis is harmful to the gums and supporting structures of the teeth (periodontium). Evidence of the association emerged from cohort studies,8,25 cross-sectional surveys,17,19 case reports,30,31 and 1 systematic review and meta-analysis.27 In these studies, cannabis smoking was an exposure and various stages of periodontal disease were outcome variables. The duration and intensity of smoking cannabis among individuals in these studies varied considerably from 2 years30 to 20 years.8
Two articles8,25 explored the association between cannabis smoking and periodontal status using data from the Dunedin (New Zealand) population birth cohort of 1972 and 1973. Meier and colleagues8 assessed the periodontal health of participants when they were 26 and 38 years old. They observed a statistically significant association between cannabis smoking and poor periodontal status (β 0.12, 95% CI 0.05–0.18) after controlling for tobacco use. They also observed that over half (55.6%) of the participants who smoked cannabis for at least 15 cumulative years were diagnosed with periodontal disease compared with 13.5% of those who never smoked cannabis. Based on these findings, the authors concluded that smoking cannabis for 20 years represented a significant risk for periodontal disease. In an earlier study based on the same cohort, Thomson and colleagues25 grouped the participants into 3 categories: no exposure, some exposure and high exposure to cannabis. The prevalence of periodontal disease was 6.5%, 11.2% and 23.6%, respectively, in the 3 groups. Even after controlling for potential confounders, people with high exposure to cannabis had a 2-fold greater chance of being diagnosed with periodontal disease (OR 1.6, 95% CI 1.2–2.2).
The results of the Dunedin birth cohort studies support those of the National Health and Nutrition Examination Survey (NHANES) in the United States.19 Compared with non-users, frequent smokers of cannabis in the NHANES study had a 40% higher chance of being diagnosed with periodontal disease (model 1: adjusted OR [aOR] 1.4, 95% CI 1.1–1.9; model 2: aOR 1.9, 95% CI 1.1–3.2), after adjustments were made for alcohol use, tobacco use and previous periodontal treatment.
Data from 2 small-scale clinical reports23,24 also indicate that frequent cannabis use is a risk factor for periodontal disease. These case reports describe localized31 and generalized gingival growth30 among frequent cannabis smokers who did not smoke tobacco or use drugs known to cause gingival growth. A combination of the clinical picture and medical history led the clinicians to suggest that cannabis smoking underpinned the periodontal condition of their clients.30
In contrast to the trend described above, a survey of Chilean adolescents aged 12–21 years, found no significant association between cannabis smoking and periodontal disease.17 The authors defined periodontal disease as the presence of necrotizing ulcerative gingival lesions or clinical attachment loss of at least 3 mm. Exploring a similar association in a birth cohort of Aboriginal children in Australia, Jamieson and colleagues24 observed a harmful association among children who reported smoking tobacco and cannabis (prevalence ratio [PR] 1.44, 95% CI 1.05–1.97). However, a sub-analysis of children who smoked cannabis exclusively was not feasible because of their small number. Thus, the study was inconclusive as to the specific association between cannabis smoking and periodontal disease.
A recently published systematic review and meta-analysis27 on cannabis and periodontal disease provided the strongest evidence between the 2 variables. After combining and analyzing data from 3 studies with adult populations and 2 studies that involved adolescent participants, the authors found a statistically significant association between smoking cannabis and periodontal disease. Compared with non-cannabis smokers, people who smoked cannabis had a 6–19% greater chance of developing periodontitis (PR 1.12, 95% CI 1.06–1.19).
Cannabis Smoking and Dental Caries
Evidence linking cannabis smoking with dental caries is sparse and inconclusive: 2 articles analyzed the association with inconsistent results. In a case–control study, Schulz-Katterbach and colleagues23 compared dental caries among 85 cannabis and tobacco smokers aged 18–25 years. They found cannabis users had significantly higher decay on the smooth surfaces of the teeth. However, caries, measured as the number of decayed and filled surfaces (DFS index) was similar for cases and controls; thus, the authors could not confirm the hypothesis that cannabis increases the risk of caries. In a second study, Ditmyer and colleagues9 analyzed trends in tobacco/cannabis use and dental caries among Nevada children and adolescents over 8 years. Compared to non-smokers, smokers of tobacco/cannabis had a significantly higher prevalence and severity of dental caries. However, the difference in caries prevalence was not significant in a sub-analysis that compared cannabis smokers to non-smokers. Among cannabis smokers, the percent mean difference (PMD) in the decayed missing and filled teeth (DMFT) fluctuated over time rather than increase progressively. The PMD was 44.9% in year 1, increased to 51.4% in year 2 ,dropped to 19.76% in year 3, before gradually increasing to 33.85% in year 8. Although the authors projected a further increase among cannabis smokers, it was difficult to attribute such changes in caries incidence or severity to the smoking of cannabis alone.
Cannabis Smoking and Oral Soft Tissues
Researchers have also been interested in the effects of cannabis smoking on oral soft tissues. Dry mouth (xerostomia) after smoking cannabis, nicotinic-like stains, leukoedema, fiery red papilloma and uvulitis are common conditions and lesions among cannabis smokers.20,21 Darling and Arendorf21 examined 300 people who used cannabis, tobacco and/or methaqualone and published an extensive list of oral soft tissue lesions that they discovered. The lesions included xerostomia, leukoedema, leukoplakia, nicotinic stomatitis, erythroplakia, denture stomatitis, Fordyce’s granules, hyperkeratosis, recurrent herpes, cheek chewer’s lesion, traumatic inflammation, traumatic ulcer angular cheilitis, median rhomboid glossitis and hypopigmented lower lip. The authors then compared the lesions of cannabis smokers and non-smokers and found those in the exposure group had a significantly higher prevalence of leukoedema, xerostomia and traumatic ulcers. In another study, Darling and colleagues20 reported significantly higher amounts of Candida albicans in the mouths of cannabis smokers compared with non-smokers. However, the prevalence and severity of oral candidiasis was similar for both groups of patients.
Cannabis Smoking and Oral Cancers
According to the literature, cannabis smoking does not significantly increase the risk of oral cancers. This conclusion is based on the results of studies that examined associations between cannabis smoking and head and neck cancers. Rosenblatt and colleagues22 explored the association between cannabis smoking and oral squamous cell carcinoma (OSCC) in a population-based case–control study that included 407 recently diagnosed patients. These patients, aged 18–65 years, were matched for age and sex with participants from the general population. Relevant data included lifetime histories of cannabis use and exposure to potential risk factors. After adjusting for sex, education, birth year, alcohol consumption and cigarette smoking, the authors found that cannabis use posed no additional risk for OSCC (aOR 0.9, 95% CI 0.6–1.3).
In a pooled analysis of 9 case–control studies, Marks and colleagues10 found cannabis smokers had a 20% higher chance of oropharyngeal carcinoma compared with non-smokers (aOR 1.24, 95% CI 1.06–1.47). However, a subgroup analysis of the association between cannabis smoking and tongue cancer suggested that smoking cannabis posed no additional risk for OSCC of the tongue (aOR 0.47, 95% CI 0.29–0.75). In fact, the association between cannabis smoking and head and neck carcinoma varied depending on the site of the tumour. Conversely, clinical reports with much smaller sample sizes32-34 suggested that smoking cannabis is a risk factor for oral cancers. The patients in these studies ranged from 18 years18 to 52 years34 and had cancers that were confirmed histologically as OSCC affecting the tongue, floor of the mouth and lips. A common feature in the medical history of these patients was the frequent smoking of cannabis for at least 5 years. The participants in these small case reports had never smoked tobacco or consumed alcohol, which are potential confounders or effect modifiers.
Cannabis Smoking, Clinical Care and Public Health
Cannabis and Clinical Care
Cannabis smoking has implications for the care of patients in dental clinics. Clinical challenges have been identified in the literature at 3 points: history taking, treatment planning and routine follow up. For example, some dentists in the United States expressed difficulties obtaining a cannabis history from patients and an inability to refer cannabis smokers who needed further support in jurisdictions where recreational cannabis is outlawed.31 Further, the altered mental state experienced after smoking cannabis impaired the ability of patients to provide informed consent.28 Faced with such a situation, some dentists postponed treatment of the patient pending further evaluation. Momen-Heravi and Kang31 enjoined dentists to watch for general and oral-health-related cannabis manifestations when providing clinical care. To ensure optimal dental care of these patients, the authors recommend a customized treatment plan that takes into account the cannabis consumption habits of the client.31 Cannabis smoking has also been shown to adversely influence the treatment of dental patients under general anesthesia. Patients who smoke cannabis within 72 h before general anesthesia experience a sustained increase in heart rate (tachycardia); this complication limits their ability to cope with post-operative stress.26
Cannabis and Dental Public Health
Evidence shows that smoking cannabis has deleterious effects on multiple human organs. The effects of frequent and long-term use of cannabis on the periodontium were described in the previous section. Further effects have been studied as well. For example, adolescents who smoke cannabis perceive that they have poor oral health and report behaviours that increase their risk of oral disease.15,16 In another study, cannabis smokers reported brushing their teeth less frequently than their non-smoking peers and failed to consult their dentist regularly.23 Cannabis smokers are also likely to be using another substance with known harmful effects, e.g., tobacco. For these people, the combined effect on oral tissues is higher than the individual risks or associations.9,22,25
Discussion
This review of evidence relating cannabis smoking and oral disease highlights the implications for clinical care and dental public health. It draws on a variety of studies that provide moderate to high levels of evidence. Based on this evidence, and in the context of the new reality of legalized cannabis in Canada, dental professionals should be alert to possible oral and general health consequences of cannabis smoking that they may encounter in their practice. For this to happen, professionals need to have an in-depth knowledge regarding medical and recreational cannabis.19
Awareness of the various forms of cannabis, modes of consumption and possible oral health effects will inform optimal dental care for cannabis smokers.1 Systematically asking the client’s history of cannabis use can help dental professionals avoid possible interactions between cannabis and drugs used in the dental clinic. In addition, this approach can help professionals explore and identify features of acute cannabis toxicity or cannabis dependence.1 Dental professionals will have to educate their clients that cannabis smoking can facilitate the onset or aggravate existing periodontal disease. Further, cannabis smokers require stricter personal oral hygiene measures and frequent visits to the dental professional for assistance and follow up.
The establishment of clinical guidelines could help dental professionals better evaluate, educate and provide high-quality oral health care for cannabis users.28,31 As more professionals start to provide care for such clients, it is important that they adopt a standard approach. In dental clinics, the “ask, advise and act” technique can enable busy professionals to provide clients with quick advice on cannabis.36 Dental professionals can also use validated instruments for rapid screening of substance use problems in clinic settings. Examples of brief self-report measures include the Drug Abuse Screening Test37 and the CAGE-AID.38 The Cannabis Use Disorders Identification Test-Revised (CUDIT-R)39 is an example of a cannabis-specific questionnaire that can guide patient education and treatment planning.
Dental professionals must be cautious when discussing the possible effects of cannabis smoking on oral disease with their clients, given the inconsistent associations between the variables (except for periodontal disease). The harmful effect of cannabis on the periodontium reported in previous articles1,40 has received validation from a recently published meta-analysis.27
Despite the evidence, the mechanism through which cannabis smoking contributes to periodontal disease is not fully understood; however, it appears to follow a dose-dependent relation, and various biological plausibilities exist for the pathophysiological changes to the periodontium. For example, the combustion temperature of cannabis smoking creates a temporary dryness of the oral tissues.41 Further, the THC in cannabis inhibits the parasympathetic nervous system, causing a reduction in the flow of saliva. Such induced xerostomia can last for up to 1h after cannabis smoking.2 A combination of increased temperature in the oral cavity and reduction in salivary flow limits the buffering capacity of saliva.42 As a consequence, the oral tissues become more vulnerable to diseases of bacterial, viral and fungal origin. This pathway can partly explain the prevalence of oral soft tissue lesions common among cannabis users. Joshi and Ashley1 have also suggested that cannabis smokers could have an increased desire for sugar-rich food and drinks, which is a risk factor for dental caries.
Evidence linking cannabis smoking and oral cancer is lacking despite the positive association with oropharyngeal cancers. This finding is counter-intuitive when one considers that cannabis and tobacco have similar carcinogenic properties. However, the THC in cannabis has been shown to have both carcinogenic and anti-carcinogenic properties, and this duality could explain why cannabis smoking shows site-specific risks for head and neck cancers.10
Evidence supports harmful effects of cannabis smoking on some oral tissues. Thus, it is prudent for dental professionals to handle cannabis smoking as a potential risk factor for poor oral health.This approach suggests that cannabis smoking be included in extant oral health prevention and promotion activities that address the harmful effects of tobacco.9
Future Directions
Further research is required to fully understand the oral health effects of cannabis smoking and the implications for the dental profession. While waiting for further evidence, dental professionals should familiarize themselves with the scientific knowledge and tools required to facilitate care for patients with acute or chronic features of cannabis smoking.
Determining the amount, frequency and duration of cannabis smoking that affects oral health is worth exploring. The legalization of recreational cannabis in Canada provides an opportunity for robust study designs (e.g., randomized controlled trials and prospective cohort studies) to answer current and emerging questions regarding cannabis and oral health.
Strength and Limitations
This study involved a comprehensive and systematic search of relevant literature from 5 electronic databases to address the research question. Two researchers independently screened the articles and extracted data to enhance rigour and minimize bias. The rapid evidence review method enabled us to answer the broad research question with findings that are relevant to clinical dental care and dental public health. Notwithstanding this careful attention, there remains a possibility that relevant articles may have been omitted. The study design is also not appropriate for combining and re-analyzing quantitative results and adjusting for the variable sample sizes of individual studies. Despite the limitations, this review is a succinct synthesis of available evidence on cannabis smoking and oral health and has the potential to inform dental professionals.
Conclusions
Cannabis smoking is harmful to the periodontium and has the potential to adversely affect care in the dental clinic. Dental professionals should be aware of these consequences and apply the knowledge in their practice. Further research is needed to fully understand the consequences of cannabis smoking on oral disease and the implications for oral health care.





