Abstract
Objectives:
This scoping review provides a comprehensive overview of oral cavity cancer (OCC) and oropharyngeal cancer (OPC) in Alberta.
Methods
A database search was conducted up to 2018 using Web of Science, Scopus, Medline, PubMed and Embase, along with a manual search of gray literature. Data from the Alberta Cancer Foundation’s dedicated fund for research, Cancer Surveillance and Reporting and Alberta Cancer Registry were also collected.
Results:
Our review included 8 published papers and 14 other sources, including data on 3448 OCC and OPC patients from Surveillance and Reporting and Alberta Cancer Registry. Cancer registry data (2005–2017) showed that most OCC and OPC lesions were diagnosed at an advanced clinical stage, with a significantly large number of advanced OPC lesions in stage IV (OCC 45.2%, OPC 82.4%); 47.9% of these patients died. Survival rates were lowest in rural and First Nations areas. In Alberta, 35% of HPV-associated cancers were linked to OPCs, which were more prevalent in men and younger age groups. No routine public oral cancer screening program currently exists in Alberta. General practitioners and dentists refer patients to specialists, often with long waiting times.
Conclusions:
OCC and OPC patients in Alberta continue to be diagnosed in stage IV and experience high mortality rates.
Oral and pharyngeal cancer remains a significant global public health issue, with about 657 000 new cases reported each year and more than 330 000 deaths.1 In 2019, 53 000 North Americans were diagnosed with oral and oropharyngeal cancer (OPC), resulting in over 9750 deaths. 2 The 2019 Canadian Cancer Statistics report estimated that 5300 Canadians will be diagnosed with oral cancer (3700 men and 1600 women), of which 1480 died (1050 men and 430 women).3 Oral cancer is 3 times more common than cervical cancer and almost twice as common as liver cancer.4 Despite existing evidence indicating that early detection of precancerous and early-stage lesions can significantly improve the survival rate and quality of life of oral cancer patients,5 3 people die from oral cancer every day in Canada.
Alberta is 4th, after Ontario, Quebec and British Columbia, in terms of oral cancer incidence and related death prevalence among Canada’s provinces and territories.3 This ranking is expected to rise, given the fast growth of the South Asian community in Alberta as the province’s second largest immigrant group. The literature has shown a high prevalence of oral cancer in this population.5,6
Oral cancer represents almost 30% of malignancies of the head and neck (H&N). The development of cancer in the oral mucosa is classified by the World Health Organization7 as a 2-step process. Oral cancer is thought to arise in premalignant lesions that undergo malignant transformation. Precancerous lesions of the mouth include leukoplakia (white patch) and erythroplakia (red patch), which are considered clinical terms. Oral cancer is more likely to occur in people with precancerous lesions than in their apparently normal counterparts.8 Unfortunately, oral cancer continues to be diagnosed mainly in advanced stages, giving patients less chance of survival.9 For decades, the survival rate for oral cancer has remained steady at 50–60%, despite several advances in cancer management.10 In India, which is well-known for its high rate of oral cancer, a study showed that prevention and early detection through visual screening of precancerous lesions dramatically decreased oral cancer mortality rates and improved quality of life in high-risk populations.5
Late detection of oral cancer can result in poor quality of life, profound psychosocial consequences and complications in the H&N area after conventional treatments, such as radical surgery, radiation therapy and chemotherapy.10 The poor prognosis is because many vital functions, including speaking, smelling, swallowing, hearing and mastication, can be seriously affected.10 Evidence has shown a strong correlation between late detection of oral cancer and poor quality of life compared with patients diagnosed in early stages.10 Moreover, early detection of oral cancer leads to treatment that is less costly for families and the health care system compared with cases diagnosed in advanced stages.11
Recent evidence has shown significant shifts in etiological factors and age groups at risk for oral cancer.12 Oral cancers have been primarily associated with tobacco and alcohol use and have been more prevalent in older age groups.13 However, increasing numbers of cases associated with human papillomavirus (HPV) occur in younger individuals.9 Analysis of social, clinical and demographic characteristics and p16 protein status of patients diagnosed with OPC at 5 Canadian cancer centres, including 2 in Alberta, showed a steady increase in HPV-associated OPCs, rising from 47.3% in 2000 to 73.7% in 2012. 14 In Alberta between 1975 and 2009, the age-standardized incidence of OPCs increased for each 5-year period by 3.4% annually among men (p < 0.001) and 1.5% among women (p = 0.009). 15
A meta-analysis of 17 studies16 showed the strongest association between HPV and tonsillar cancer, an intermediate association with OPC and the weakest link with oral cancer. Oropharyngeal cancer as an additional entity, can be screened by dentists/dental hygienists where possible, through a careful examination of the soft palate, tonsils and neck.
Oral cavity cancer (OCC) and OPC are deadly diseases, particularly in stages III and IV. However, the survival rate is more than 80% for patients diagnosed in stages I or II.17 Both diseases continue to be diagnosed at advanced stages even though, in most cases, they could easily be detected visually by health professionals, especially dentists and family physicians. The literature describes 2 distinct categories of delays: “patient delay” or time from the patient’s awareness of changes to her/his presentation to health professionals and “professional delay” or time from patient’s presentation to a heath care provider to definitive diagnosis and treatment (Fig. 1). 18 If left untreated, 5% of leukoplakia and 50% of erythroplakia can develop into oral cancer.19 Early clinical detection of oral lesions and confirmation of premalignant status (thus facilitating timely treatment) could prevent the development of aggressive malignancies. A comprehensive investigation is required to unfold how and where suspicious lesions are ignored. The sooner a patient with oral cancer is identified, diagnosed and given initial treatment, the better their chance of survival.
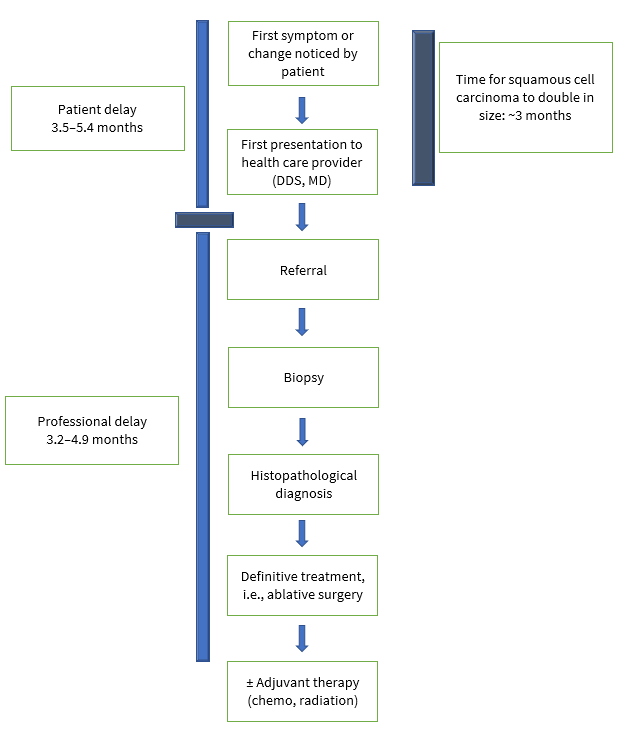
Figure 1: Two categories of delay in diagnosis of oral cancers: patient and professional.18
The statistical evidence regarding OCC and OPC incidence and related projected death rates in Alberta is alarming: an increase of 76.1% in new cases of OCC and OPC is expected between 2003–07 and 2028–32.20 Few peer-reviewed studies show the status of OCC and OPC in the province, and the most important related data are scattered across a number of governmental and nongovernmental institutions and organizations. Therefore, the objectives of this scoping review were to investigate:
- The prevalence of OCC and OPC in Alberta according to patients’ demographics and tumour characteristics
- The usual route from detection of OCC and OPC to treatment
- Existing OCC and OPC prevention initiatives
- Funding of OCC and OPC prevention
- Where and by whom patients with OCC and OPC are initially diagnosed
A preliminary search for scoping reviews on this topic was conducted at Web of Science, Scopus, Medline, PubMed, Embase, the Joanna Briggs Institute Database of Systematic and Implementation Reports and the Cochrane Database of Systematic Reviews, but no related review was found.
Materials and Methods
Our protocol was developed based on the methodological framework for scoping studies proposed by Arksey and O’Malley.21 It consists of 5 stages: identifying research questions and objectives; identifying relevant studies based on inclusion criteria; selecting studies; charting the data; and collating, summarizing and reporting the results. Ethics approval for data extraction from the Alberta Cancer Registry was obtained from the Health Research Ethics Board of Alberta’s Cancer Committee.
Research question: What is the prevalence, demographics, initial diagnosis, prevention, management and research funding allocated for early detection of OCC and OPC in Alberta?
Inclusion Criteria
This scoping review included adults aged 18 years and older living in Alberta.
Provincial OCC and OPC data are reported in diverse media, and our search strategy aimed to gather data from as many resources as possible. These included peer-reviewed, published, unpublished and hand-searched gray literature (e.g., primary research studies, systematic reviews, letters, guidelines, Google and Google Scholar). In addition, the search included governmental and nongovernmental institutions and organizations.
A 3-step search strategy22 was performed for the timeframe 1990–2018. We used all key terms to cross-search all databases, including Web of Science, Scopus, Medline, PubMed and Embase. We also conducted a search of the Joanna Briggs Institute Database of Systematic Reviews and Implementation Reports and the Cochrane Database of Systematic Reviews to retrieve potential similar published reviews. Keywords were ((mouth or oral or gingiv* or lip or lips or palat* or tonsil or parotid or sublingual or lingual or tongue or cheek*) and (cancer or neoplasm* or tumor* or tumour* or malignan* or carcinoma*)) AND TOPIC: (alberta or calgary or edmonton).
We also conducted a hand-search of gray literature and used Google and Google Scholar search engines to find relevant articles. Finally, we emailed and telephoned Alberta Cancer Foundation Surveillance and Reporting, Alberta Prevents Cancer, Alberta Health Services and Alberta Cancer Registry to gather relevant information uncovered by the search engines.
Results
Study Selection
Published literature
The search strategy resulted in identification of studies from Medline (n = 48), Embase (n = 74), Pubmed (n = 33), Scopus (n = 74) and Web of Science (n = 55) for a total of 284. Of these, 153 were eliminated because of duplication. Two reviewers excluded 96 irrelevant studies based on title and abstract. After reviewing the full text, 25 more studies were screened and excluded as they did not meet the inclusion criteria of the study. A final set of 8 studies23-30 were included in this review (Fig. 2).31
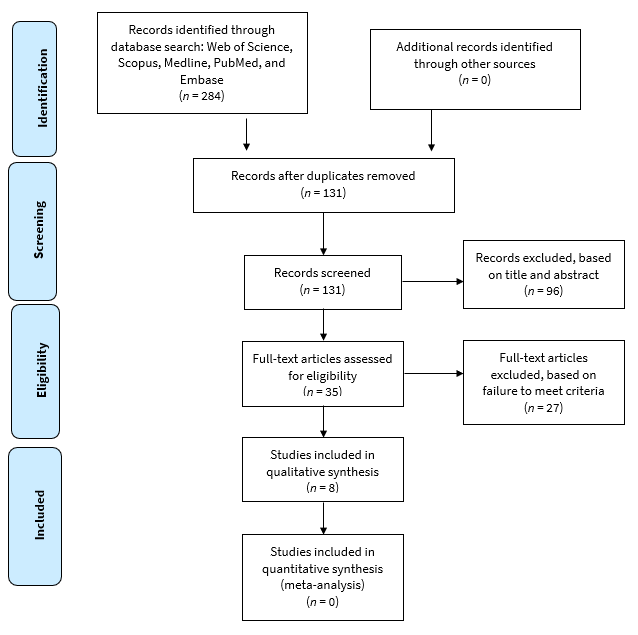
Figure 2: PRISMA process for selection of records for review.31
Gray literature
Relevant thesis monographs (n = 2)32,33 and clinical guidelines for H&N cancer delivery and management in Alberta (n = 2)34,35 were identified and included in the study. Other sources were Canadian Cancer Statistics for 2015–2017,20,36,37 the Alberta Cancer Foundation,38 data on 3448 patients from the Surveillance and Reporting–Alberta Cancer Registry, Alberta Health Services,39-41 Alberta Innovates,42 Canadian Cancer Society and Alberta Cancer Prevention Legacy Fund (ACPLF)43 and Canadian Institutes of Health Research.44
Charting the Data
The information that was relevant to our study objectives, including descriptive and numeric data, reports and chart information on patients, was extracted and charted according to Joanna Briggs Institute guidelines.22 For the published studies, extracted data included: author(s)/year/country of origin; aims; population/characteristics/size; study design; concepts relevant to our study objectives; context relevant to our study objectives; and outcome relevant to our study objectives.
Of the 8 published studies, 7 were quantitative,23-29 and 1 was qualitative.30 All were conducted in Alberta and published between 2004 and 2016. They focused on care plans and quality management of OCC and OPC,30 factors influencing survival,23-25 comorbidity and risk management26,27 and the epidemiological evolution of HPV associated with OCC and OPC.28,29 Detailed characteristics of these studies are presented in Table 1.
The 2 master’s theses reported on quality of life, especially for young patients diagnosed with OCC and OPC in Alberta,32 and referral patterns of patients to the University of Alberta oral medicine clinic, a specialty clinic where dentists refer patients with suspicious oral lesions to be evaluated by certified specialists in oral medicine and pathology in Edmonton.33 Detailed data from gray resources are presented in Tables 2–6 and Figure 3.

Figure 3: Trends in the incidence of oral cavity cancer (OCC) and oropharyngeal cancer (OPC), based on data from the Alberta Cancer Registry, 2005–2017.
|
Author(s) and publication year |
Aim of study |
Study population: characteristics/size |
Study design |
Concepts relevant to our study objectives |
Contexts relevant to our study objectives |
Duration of study |
Outcomes relevant to our scoping review questions |
|---|---|---|---|---|---|---|---|
|
Note: H&N = head and neck, HPV = human papillomavirus, NA = not applicable, OCSCC = oral cavity squamous cell carcinoma, OPC = oropharyngeal cancer, OPSCC = oropharyngeal squamous cell carcinoma. |
|||||||
| Biron et al. 201323 | To evaluate disparities in clinical vs. pathological TNM staging in OCSCC patients and any impact of this on survival | All patients undergoing surgical treatment for OCSCC in Alberta | Quantitative | Staging, diagnosis, management | Survival pathway | 1998–2006 | Some disparity exists in clinical vs. pathological staging in OCSCC; however, this does not have any significant impact on disease-specific survival |
| Zang et al. 201524 | To investigate the association between survival and geographic location | 554 charts of patients diagnosed with OCSCC in Alberta | Quantitative | Geographic, demographic | Survival pathway | 1998–2010 | Patients from urban locations had improved overall, disease-specific and disease-free survival compared with those in rural locations |
| Erickson et al. 201525 | To assess First Nations survival trends | 583 patient records from Alberta Cancer Registry;19 First Nations were included | Quantitative | Ethnicity, demographics | Survival pathway | 1998–2009 | Survival and disease-specific survival were significantly lower in First Nations patients compared with other patients with OCSCC |
| Thompson et al. 200426 | To determine the incidence of perioperative stroke in patients undergoing neck dissection | 499 records of discharge data for all neck dissections performed in a geographically defined health region in Alberta | Quantitative | Perioperative risk assessment for stroke incidence | Oral cancer quality management | 1994–2002 | Incidence of perioperative stroke in this study was significantly lower than previously stated in the literature; suggests that preoperative screening and/or intervention for carotid artery disease may not be necessary in this patient population |
| Barber et al. 201527 | To assess depression as a predictor of postoperative functional performance status and treatment adherence in H&N cancer patients | All new adult patients with H&N cancer undergoing surgery as primary therapy | Quantitative | Post -operative functional assessment in patient with preoperative depressive symptoms. | Oral cancer quality management | May 2013 to January 2014 | Incidence and severity of preoperative depressive symptoms in H&N cancer patients treated with surgery was high (53.5 %) |
| Shack et al. 201428 | To assess temporal, age-specific and sex-specific changes in the incidence of noncervical and cervical cancers associated with HPV in a population-based study | Identified 8120 HPV-associated cancer records from Alberta Cancer Registry among all cancers diagnosed in the province, targeting patients with cancers of the oropharynx, cervix, vulva, vagina, anus and penis | Annual % change using joinpoint regression | New HPV-associated OPC trend | Oral cancer based on age and sex in younger cohort | 1 Jan. 1975 to 31 Dec. 2009 | Increased incidence of HPV-associated cancers of the oropharynx and anus among men and women and increase in cervical cancer among younger women |
| Marzuki et al. 201629 | To investigate the possible epidemiological association between oropharyngeal carcinoma and anogenital tumours | 2015 male patients diagnosed with OPSCC and anogenital cancer in Alberta | Quantitative | HPV epidemiologic pathway | Lifestyle diversity | 1980–2011 | No significant risk factors for anogenital cancer associated with OPSCC |
| Collie et al. 201430 | To evaluate care plans for H&N cancer survival | 21 H&N cancer survivors in Alberta | Quantitative | Quality of care and treatment | Survival and quality management | NA | Care plans could help to improve the transition to cancer survivorship |
|
|
Grattan 201332 |
Friesen 201833 |
|||||
|---|---|---|---|---|---|---|---|
|
Note: H&N = head and neck, HPV = human papillomavirus, NA = not applicable, OPC = oropharyngeal cancer. |
|||||||
| Aim of study | Evaluation of physical, psychosocial, and sexual quality of life for young H&N patients | Assessment of referral patterns to an oral medicine clinic at the University of Alberta | |||||
| Study population:characteristics/size | Ten H&N patients, aged 18-65 years | Review of 924 patients’ charts | |||||
| Study design | Mixed methods | Quantitative | |||||
| Concepts relevant to our study objectives | HPV as new recognized risk factor associated with oral cavity and oropharyngeal cancer in younger age | Pathway referrals and efficacy of management services | |||||
| Context relevant to our study objectives | Diverse aspects of quality of life, mostly for young H&N cancer patients infected with HPV | Demographic, distance travel to the centre, wait time from referral to first appointment for suspicious oral lesions | |||||
| Duration of study | NA | 1 year (2015) | |||||
| Outcome relevant to our scoping review questions | Recent trend in association between HPV and OPC in younger individuals and the special burden of quality of life vs that of older patients | Of 924 patients with suspicious oral lesions, 361 (39%) were male and 563 (61%) were female Average distance 55.44 km; median 16.60 km; maximum 2028.00 km; minimum 1.40 km Patient wait time: 100 patients 0–30 days; 340 patients 31–90 days; 484 patients > 90 days |
|||||
| Study research question: What is the pattern of referral to an oral medicine clinic at the University of Alberta? | General dentist 688 referrals (74.5%); dental specialists 64 referrals (6.8%); total 752 referrals (81.4%) Family physician 146 referrals (15.8%); dermatologist 16 referrals (1.7%); ear, nose and throat 8 (0.8%); rheumatologist 2 (0.2%); total 172 referrals (18.6%) |
||||||
|
Variable |
Category |
OCC patients (%) |
OPC patients (%) |
||||
|---|---|---|---|---|---|---|---|
|
* Based on household income of patients at diagnosis. |
|||||||
| Sex | Male | 1026 (58.2) | 1377 (81.7) | ||||
| Female | 737 (41.8) | 308 (18.3) | |||||
| Total | 1763 (100) | 1685 (100) | |||||
| Age at diagnosis, years | ≤ 45 | 166 (9.4) | 98 (5.8) | ||||
| 46–65 | 799 (45.3) | 1139 (67.6) | |||||
| > 65 | 799 (45.3) | 448 (26.6) | |||||
| Total | 1764 (100) | 1685 (100) | |||||
| Average income* | < 45 000 | 447 (25.4) | 411 (24.4) | ||||
| 45 000–75 000 | 779 (44.2) | 664 (39.4) | |||||
| > 75 000 | 537 (30.5) | 610 (36.2) | |||||
| Total | 1763 (100) | 1685 (100) | |||||
| Region | Rural | 677 | 17.5 | ||||
| Urban | 3188 | 82.5 | |||||
| Total | 3865 | (100) | |||||
| Diagnosis location | Z1 (South Alberta) | 132 (7.5) | 127 (7.5) | ||||
| Z2 (Calgary) | 663 (37.6) | 628 (37.3) | |||||
| Z3 (Central Alberta) | 236 (13.4) | 206 (12.2) | |||||
| Z4 (Edmonton) | 572 (32.4) | 536 (31.8) | |||||
| Z5 (North Alberta) | 160 (9.1) | 188 (11.2) | |||||
| Total | 1763 (100) | 1685(100) | |||||
| Survival status | Alive | 818 (46.4) | 977 (58.3) | ||||
| Dead | 945 (53.6) | 707 (41.7) | |||||
| Total | 1763 (100) | 1672(100) | |||||
| Age at death | ≤ 45 | 25 (2.6) | 9 (1.3) | ||||
| 46–65 | 278 (29.4) | 319 (45.1) | |||||
| > 65 | 642 (67.9) | 379 (53.6) | |||||
| Total | 945 (100) | 707 (100) | |||||
|
Cancer site |
Males (%) |
Females (%) |
Total (%) |
|---|---|---|---|
| Base of tongue | 546 (32.40) | 96 (5.70) | 642 (38.10) |
| Floor of mouth | 184 (10.44) | 94 (5.33) | 278 (15.77) |
| Gum | 102 (5.79) | 86 (4.88) | 188 (10.66) |
| Lip | 52 (2.95) | 18 (1.02) | 70 (3.97) |
| Lip, oral cavity and pharynx, other and unspecified | 11 (0.65) | 2 (0.12) | 13 (0.77) |
| Oropharynx | 154 (9.14) | 50 (2.97) | 204 (12.11) |
| Mouth, other and unspecified | 172 (9.76) | 126 (7.15) | 298 (16.90) |
| Palate | 79 (4.48) | 81 (4.59) | 160 (9.08) |
| Tongue, other and unspecified | 447 (57.30) | 333 (42.70) | 780 (78.91) |
| Tonsil | 656 (38.93) | 159 (9.44) | 1685 (48.37) |
|
Tumour stage (n = 3448) |
OCC cases (%) |
OPC cases (%) |
Total |
|---|---|---|---|
| I | 446 (29.6) | 41 (2.6) | 487 |
| II | 221 (14.6) | 78 (5.0) | 299 |
| III | 151 (10.0) | 158 (10.0) | 309 |
| IV | 691 (45.8) | 1296 (82.4) | 1987 |
| Total | 1509 (100) | 1573 (100) | 3082 |
| Unknown | 234 | 112 | 346 |
| *Total Missing Data: 20 | |||
|
Research question |
Data |
||||||
|---|---|---|---|---|---|---|---|
|
Note: HPV = human papillomavirus, OCC = oral cavity cancer, OPC = oropharyngeal cancer. |
|||||||
| 1 | What is the prevalence of OCC and OPC in Alberta?* | OCC and OPC increased in prevalence between 2005 and 2017. In 2005–2017, of 3448 cases, 1763 (51%) were diagnosed as OCC and 1685 (48.8 %) as OPC. OCC and OPC were more prevalent in men and occurred at a younger age, with a higher rate for oropharyngeal cancers (81.7) versus OCC (58.2%). The percentage of patients diagnosed with OCC and OPC was higher in urban (82.5%) versus rural (17.5%) areas. Survival was lowest in rural and First Nations communities.25 Deceased (2005–2017): OCC 945 (27.4%); OPC = 707 (20.5%); total 1652 (47.9%). Stage IV (2005–2017): OCC 691(45.19%); OPC 1296 (82.39%); total 1987. |
|||||
| 2 | What is the ongoing OCC and OPC prevention strategy in Alberta? | Other than HPV vaccination of young men and women since 2008, there are no preventive or routine oral/head and neck cancer screenings in place in Alberta. Note: HPV-9 vaccine is up to 99% effective in preventing HPV-related disease from the 9 HPV strains including 25% of H&N cancers.41 | |||||
| 3 | What funds are allocated for OCC and OPC in Alberta? | The funds are mainly allocated for treatment and therapy, targeting improving quality of life rather than prevention of disease38,43,44. | |||||
| 4 | What are the pathways for OCC and OPC in Alberta? | “Treatment algorithms” presented by Alberta Health Services:
|
|||||
Collating, Summarizing and Reporting the Results
In Tables 1–6, information is classified according to the objectives of the study and it provides an overview of factors associated with OCC and OPC in Alberta.
Tumour location/site was categorized according to the topographical codes in the International Classification of Diseases for Oncology, 3rd edition, ICD-0 3. OCC sites included lip (C00.3-C00.9), oral tongue (C2.0-C2.3, C2.8 and C2.9), gum (C3.0-C3.0), floor of mouth (C4.0-C4.9), palate (C5.0-C5.9) and other and unspecified parts of the mouth (C6.0-C6.9). OPC sites included base of tongue (C01), lingual tonsil (C2.4), tonsil (C9.0-C9.9), oropharynx (C10.0-C10.9), pharynx not otherwise specified (C14.0) and Waldeyer ring (C14.2). External upper and lower lip (C00.0-C00.1), parotid gland (C07.9) and other and unspecified major salivary gland tumours (C08.0-C08.9) were excluded.
According to 2005–2017 Alberta Cancer Registry data, 45.2% of OCC patients and 82.4% of OPC patients were diagnosed at stage IV, of which 47.9% died (OCC 27.41%, OPC 20.05%). The tonsils, tongue and base of tongue were the main locations of these cancers. The increased incidence of HPV-associated OPC is most striking in males < 45 years of age.36 Although most mouth and throat cancers were primarily associated with tobacco and alcohol use, about 25–35% of OCCs and OPCs were attributed to high-risk HPV types.37
Referrals and access to clinical specialists for patients with suspicious oral lesions were evaluated and reported.33 The waiting time between receiving a referral and seeing a specialist was 105.5 days on average, with a maximum of 905 days. The reported travel distance was 55.44 km on average, with a maximum of 2028 km. In this study,33 general dentists (74.5%) were found to be the main source of referrals to oral pathologists, oral surgeons and otolaryngologists, followed by family physicians (15.8%). Of all referrals, 38% were diagnosed with malignant and premalignant lesions, which represents the highest percentage among all conditions requiring the care of a specialist.33 However, according to Alberta Cancer Registry data, otolaryngologists were the most common clinicians referring OCC and OPC patients to oncologists (cancer care) followed by surgeons (general, oral, and thoracic) and general practitioners with dentists being in 8th place.
In general, management was guided by 2 sources.34,35 The recommended guidelines for H&N cancer in Alberta were adapted by the executive of the Alberta Provincial Head and Neck Tumour Team using “The Management of Head and Neck Cancer in Ontario: Section 1, Organizational and Clinical Practice Guideline Recommendations.”45 The guideline considers H&N cancer a complex chronic disease that should be managed by a qualified team with particular recommendations for: health care team components; minimum cancer centre and team member volumes; infrastructure; and waiting time. The practice guideline will be updated at least annually with any new evidence or contextual information. The latest guidelines for OCC (2014) and OPC (2019) are presented in Table 6.
Oral cancer survival was explored in 4 recent studies.23-25,30 They found that geographic location,24 ethnicity,25 quality of management30 and disparities in clinical versus pathological staging diagnosis23 could affect survival outcomes.
Pre- and post-operative risk assessments and quality of life were investigated in 3 studies.26-28 Although the findings of 1 study26 indicated that the risk of stroke in patients undergoing neck dissection surgery is low and there is no need for pre-operative screening, another study28 showed that there was a higher risk for hypothyroidism after radiation therapy in OCC and OPC patients. Thus, patient screening was recommended to achieve a higher quality of care. In addition, severity of depression was identified as a predictor of post-operative functional performance, quality of life and adherence to treatment.28
We did not identify any H&N cancer screening programs in Alberta. However, preventive HPV vaccination was approved for females aged 9–26 in 2006 and males aged 9–26 in 2012 to prevent 95% of cervical cancers and 25% of OPC.36 Furthermore, multiple websites offer intraoral cancer information and list risk factors for developing oral cancer and preventive recommendations for the public.38-41 The resources of multiple health funding agencies37,41-43 are dedicated mostly to OCC and OPC treatment studies and research investigations that target better post-treatment quality of life for OCC and OPC cancer patients rather than studies aimed at identifying effective preventive strategies for those cancers in Alberta.
Discussion
According to the Alberta Cancer Registry data from 2005 to 2017, although the incidence of OCC increased somewhat, the number of OPC cases increased significantly. In our study, the numbers of OCC and OPC cases included were almost equal (51% and 49%, respectively). Although male predominance was found for both OCC and OPC, the gender difference was larger for OPC (82.7% males among OPC cases vs. 58.2% for OCC).
This scoping review provides an overview of OCC and OPC in Alberta based on various data sources distributed across multiple bodies. Our finding is in agreement with the recent literature that HPV- associated OPCs occur in younger people compared to those diagnosed with oral cancers associated with smoking and alcohol consumption.
Although we found no published studies on strategies for preventing OCC, we did note that the upward trend in HPV-associated OPC has received significant attention. Canadian Cancer Statistics 2016, which was dedicated to HPV-associated cancers, stated that the annual number of OPC cases (for both sexes combined) is already rivaling cervical cancer in Canada.36 That report predicted that the age-standardized incidence of OPC in males may surpass that of cervical cancer in females in the near future, which is alarming. A study conducted by Shack and colleagues28 confirmed this trend in Alberta.
Following the lead of Prince Edward Island, Alberta has introduced a public health policy46 to vaccinate young people of both sexes, 9‑26 years old, to prevent HPV-associated cervical cancer. However, effective preventive strategies for non-HPV-associated OCC and OPC — e.g., regular oral/H&N cancer screening — are still lacking in Alberta, even for populations with higher vulnerability, such as those who are socioeconomically disadvantaged and/or exposed to tobacco, alcohol and recreational drugs.
The findings of this study indicate that, as in Ontario (2003–2013),47 men in Alberta are at higher risk for OCC and OPC. However, a retrospective study conducted in British Columbia analyzing OCC and OPC in 1981–2020 shows that OCC incidence is decreasing in men and increasing in women, while rates for OPC are increasing in both men and women.48
In contrast, new evidence points to an increasing incidence of OCC among Caucasian women in the United States (1973–2012), rising to the same level as men.49 This might be explained by recent changes in lifestyle. The trend also accords with the findings of a study that investigated recent OCC and OPC data globally.50
Our study shows that, as of 2017, Alberta is 4th in terms of new cases of OCC and OPC (430) behind Ontario (1950), Quebec (1070) and British Columbia (600).37 Compared with earlier data, these numbers are rising, except for a slight decrease in British Columbia.48 The large number of South Asian and Chinese ethnic minorities, known for their high incidence of OCC (South Asians)6 and OPC (Chinese),48 could explain the high rates and increases in the disease in Ontario, British Columbia and Alberta. This shows that ethnic-associated practices, such as particular smoking habits, are risk factors for OCC and OPC, despite current geographic location.48,51 Further investigation is needed to identify potential risk factors in Quebec.
Our findings for Alberta are in accord with worldwide reports and confirm the lack of improvement in terms of delays in diagnosis and survival of OCC and OPC patients, as found in the literature. This continues to be a tremendous concern for health care providers and health authorities. From 2005 to 2017, more than half the accumulated cases of OCC and OPC were diagnosed in stage IV, and 47.9% of all patients died. This finding is striking and deserves attention. There is a lack of data on prevalence of stage IV OCC and OPC cancers in the general population in Canada. Analysis of various datasets suggests that the assessment section of Alberta’s guidelines needs further investigation of the gaps identified in this scoping review regarding late access to initial clinical assessment32 and late diagnosis of OCC34 and OPC.35
This study found that the treatment and management of OCC (2014)34 and OPC (2019)35 in Alberta is guided by the Alberta Health Services H&N cancer guidelines. However, there is a lack of emphasis on the efficacy of the practice algorithm for patients, including the required waiting time for each step.
Suspected premalignant white and red oral lesions are the most common reason for referral of patient to specialists, and our findings highlight a long wait time for these patients.32 This deserves further attention as these lesions have a high potential of becoming cancerous in an interval of 1–30 year time period (leukoplakia 0.1%–36% and erythroplakia 14%–50% chance of malignant transformation).19 Reducing wait times and facilitating access for patients in remote areas to competent oral health care professionals, including oral medicine specialists, oral pathologists, oral surgeons and otolaryngologists/ear, nose and throat specialists, could improve the detection of these lesions at an earlier stage. Earlier access can have a tremendous impact on care and regular follow-ups, which, in turn, would lead to a much better treatment outcome. Friesen’s study33 also indicates the need to enhance dentists’ and physicians’ skills in the initial assessment of oral precancerous lesions. This finding has been confirmed by other studies worldwide.52,53
Multiple governmental and nongovernmental organizations and websites provide information to raise awareness about OCC and OPC and preventive instructions. Although this information could contribute to better public knowledge, no studies have evaluated their effectiveness in preventing OCC and OPC in Alberta.
Strengths and Limitations
This study included every category of data source to answer comprehensive objectives. However, in a scoping review, no quality assessment is provided, mainly because of the diversity of sources.22
Conclusions
In Alberta, the high prevalence of stage IV OCC and OPC and the associated mortality rate indicate an urgent need to investigate strategies that may improve the detection, diagnosis and management of these diseases across the province. Some suggestions include public awareness about signs and symptoms of OCC and OPC and when to approach a physician or dentist. Barriers, such as long waiting lists for first visits and long travel distances to specialized health care centres, should also be addressed. Also, implementing routine oral/H&N cancer screening in public settings, especially in at-risk communities, may lead to early detection and, consequently, better outcomes in the management of OCC and OPC in Alberta.
Implications for Research
This study shows significant evidence of late diagnosis of OCC (45.2% of patients diagnosed at stage IV) and OPC (82.4%) in Alberta between 2005 and 2017. In-depth qualitative analysis of initial consultation letters of oral cancer patients and interviewing health care providers and patients may help us understand the knowledge gap causing continued late detection. In addition, exploration of the following identified knowledge gaps could generate a better picture of the shortcomings of oral cancer diagnoses in Alberta.
- Is poor coordination between dentists and physicians to blame for the high numbers of stage IV OCC and OPC in Alberta?
- Are dentists sufficiently trained to detect and diagnose premalignant and malignant oral lesions and refer patients with these conditions to the appropriate specialists?
- What is the reason for the lack of dedicated research funding for oral cancer prevention or early-stage detection?
- How is Alberta doing relative to other jurisdictions in Canada and globally? Are there other areas in Canada or in the rest of the world with lower prevalence of stage IV OCC and OPC cases? If so, how was this accomplished?
Addressing the latter research questions requires conducting national/international multi-institutional collaborative studies to generate data and identify the prevalence of oral cancer stages for each jurisdiction and location.
Implications for Practice
Our findings identified some structural barriers to care for patients with premalignant and malignant oral lesions, including long wait times and transportation issues. Ultimately, addressing barriers and facilitating access to care for potential OCC and OPC patients could result in earlier cancer detection and thus have a crucial impact on turning a dismal outcome associated with stage IV cancer into much-improved survival resulting in better quality of life.
THE AUTHORS
Corresponding author: Dr. Maryam Amin, Faculty of Medicine and Dentistry, University of Alberta, 5513-476 Edmonton Clinic Health Academy, 11405–87 Avenue NW, 5 Floor, Edmonton AB T6G 1C9. Email: maryam.amin@ualberta.ca
Acknowledgements: This study was supported by Alberta Innovates — Health Solutions. We thank Dr. Rafael Figueiredo, Provincial Dental Public Health Officer, Alberta Health Services, for his contribution to the study. We also acknowledge the contributions of senior librarian Linda Slater who assisted in the study by developing the search terms and strategy.
The authors have no declared financial interests.
This article has been peer reviewed.
References
- Oral cancer. Geneva: World Health Organization; n.d. Available: https://www.who.int/cancer/prevention/diagnosis-screening/oral-cancer/en/ (accessed 2020 May 23).
- Oral and oropharyngeal incidence rates for 2019. Newport Beach, Calif.: Oral Cancer Foundation; 2017. Available: https://oralcancerfoundation.org/oral-oropharyngeal-incidence-rates-2017/ (accessed 2019 Jan. 24).
- Canadian Cancer Statistics Advisory Committee. Canadian cancer statistics 2019. Toronto: Canadian Cancer Society; 2019. Available: http://cancer.ca/Canadian-Cancer-Statistics-2019-EN (accessed 2020 Jan. 29).
- Oral cancer. Ottawa: Government of Canada; rev. 2019. Available: https://www.canada.ca/en/public-health/services/oral-diseases-condition… (accessed 2018 Jan. 28).
- Sankaranarayanan R, Ramadas K, Thomas G, Muwonge R, Thara S, Mathew B, et al. Effect of screening on oral cancer mortality in Kerala, India: a cluster-randomised controlled trial. Lancet. 2005;365(9475):1927-33.
- Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009;45(4-5):309-16.
- A digital manual for the early diagnosis of oral neoplasia. Geneva: World Health Organization. 2021. Available: https://screening.iarc.fr/atlasoral_list.php?cat=A&lang=1 (accessed 23 Feb 2021)
- Napier SS, Speight PM. Natural history of potentially malignant oral lesions and conditions : an overview of the literature. J Oral Pathol Med. 2008;37(1):1-10.
- Epstein JB, Huber MA. The benefit and risk of screening for oral potentially malignant epithelial lesions and squamous cell carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;120(5):537-40.
- LeHew CW, Epstein JB, Kaste LM, Choi Y-K. Assessing oral cancer early detection: clarifying dentists’ practices. J Public Health Dent. 2010;70(2):93-100.
- Laronde DM, Hislop TG, Elwood JM, Rosin MP. Oral cancer: just the facts. J Can Dent Assoc. 2008;74(3):269-72.
- Isayeva T, Li Y, Maswahu D, Brandwein-Gensler M. Human papillomavirus in non-oropharyngeal head and neck cancers: a systematic literature review. Head Neck Pathol. 2012;6(suppl 1):S104-S20.
- Denson L, Janitz AE, Brame LS, Campbell JE. Oral cavity and oropharyngeal cancer: changing trends in incidence in the United States and Oklahoma. J Okla State Med Assoc. 2016;109(7-8):339-45.
- Habbous S, Chu KP, Lau H, Schorr M, Belayneh M, Ha MN, et al. Human papillomavirus in oropharyngeal cancer in Canada: analysis of 5 comprehensive cancer centres using multiple imputation. CMAJ. 2017;189(32):E1030-40.
- Shack L, Lau HY, Huang L, Doll C, Hao D. Trends in the incidence of human papillomavirus–related noncervical and cervical cancers in Alberta, Canada: a population-based study. CMAJ Open. 2014;2(3):E127-32.
- Hobbs CGL, Sterne JAC, Bailey M, Heyderman RS, Birchall MA, Thomas SJ. Human papillomavirus and head and neck cancer: a systematic review and meta‐analysis. Clin Otolaryngol. 2006;31(4):259-66.
- Ribeiro IP, Barroso L, Marques F, Melo JB, Carreira IM. Early detection and personalized treatment in oral cancer: the impact of omics approaches. Mol Cytogenet. 2016;9:85.
- Stefanuto P, Doucet JC, Robertson C. Delays in treatment of oral cancer: a review of the current literature. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;117(4):424-9.
- Queiroz SIML, Costa de Medeiros AM, Pereira da Silva JS, Dantas da Silveira ÉJ. Clinical and histopathological evaluation and habits associated with the onset of oral leukoplakia and erythroplakia. J Bras Patol Med Lab. 2014;50(2):144-9.
- Canadian Cancer Society’s Advisory Committee on Cancer Statistics. Canadian cancer statistics 2015: special topic: predictions of the future burden of cancer in Canada. Toronto: Canadian Cancer Society; 2015. Available: https://www.cancer.ca/~/media/cancer.ca/CW/cancer%20information/cancer%20101/Canadian%20cancer%20statistics/Canadian-Cancer-Statistics-2015-EN.pdf?la=en (accessed 2021 Feb. 11).
- Arksey H, O'Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Method. 2005;8(1):19-32.
- Peters MDJ, Godfrey CM, McInerney P, Baldini Soares C, Khalil H, Parker D. The Joanna Briggs Institute reviewers' manual 2015: methodology for JBI scoping reviews. Adelaide, Australia: Joanna Briggs Institute; 2015.
- Biron VL, O'Connell DA, Seikaly H. The impact of clinical versus pathological staging in oral cavity carcinoma — a multi-institutional analysis of survival. Otolaryngol Head Neck Surg. 2013;42(1):28.
- Zhang H, Dziegielewski PT, Jean Nguyen TT, Jeffery CC, O’Connell DA, Harris JR, et al. The effects of geography on survival in patients with oral cavity squamous cell carcinoma. Oral Oncol. 2015;51(6):578-85.
- Erickson B, Biron VL, Han Z, Seikaly H, Côté DWJ. Survival outcomes of First Nations patients with oral cavity squamous cell carcinoma (Poliquin 2014). Otolaryngol Head Neck Surg. 2015;44(1):1-5.
- Thompson SK, Southern DA, McKinnon JG, Dort JC, Ghali WA. Incidence of perioperative stroke after neck dissection for head and neck cancer: a regional outcome analysis. Ann Surg. 2004(3):428-31.
- Barber B, Dergousoff J, Nesbitt M, Mitchell N, Harris J, O'Connell D, et al. Depression as a predictor of postoperative functional performance status (PFPS) and treatment adherence in head and neck cancer patients: a prospective study. Otolaryngol Head Neck Surg. 2015;44:38.
- Shack L, Lau HY, Longlong H, Doll C, Hao D. Trends in the incidence of human papillomavirus-related noncervical and cervical cancers in Alberta, Canada: a population-based study. CMAJ Open. 2014;2(3):E127-32.
- Marzouki HZ, Biron VL, Harris J, O’Connell D, Seikaly H. Human papillomavirus-associated oropharyngeal squamous cell carcinoma and anogenital cancers in men: epidemiologic evaluation of association. Head Neck. 2016;38(suppl. 1):E2100-2.
- Collie K, McCormick J, Waller A, Railton C, Shirt L, Chobanuk J, et al. Qualitative evaluation of care plans for Canadian breast and head-and-neck cancer survivors. Curr Oncol. 2014;21(1):e18-28.
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264-9.
- Grattan KS. The experiences of the younger head and neck cancer client. Master’s thesis. Edmonton: University of Alberta; 2013.
- Friesen R. A retrospective analysis of referral patterns to a university oral medicine clinic. Master’s thesis. Edmonton: University of Alberta; 2018.
- Team APHaNT. Clinical practice guideline: HN-002 version 1. Edmonton: Alberta Health Services; 2014. Available: https://pdfslide.net/documents/oral-cavity-cancer-alberta-health-2016-06-23developing-oral-cavity-cancer-oral.html (accessed 2018 Feb. 20).
- Oropharyngeal cancer treatment: clinical practice guideline HN-004 version 1. Edmonton: Alberta Health Services; 2019. Available: https://www.albertahealthservices.ca/assets/info/hp/cancer/if-hp-cancer-guide-hn004-oropharyngeal.pdf (accessed 2020 February 10).
- Canadian Cancer Society’s Advisory Committee on Cancer Statistics. Canadian cancer statistics 2016 — special topic: HPV-associated cancers. Toronto: Canadian Cancer Society; 2016. Available: http://www.cancer.ca/~/media/cancer.ca/CW/cancer%20information/cancer%20101/Canadian%20cancer%20statistics/Canadian-Cancer-Statistics-2016-EN.pdf?la=en (accessed 2018 February 27).
- Canadian Cancer Society’s Advisory Committee on Cancer Statistics. Canadian cancer statistics 2017 — special topic: pancreatic cancer. Toronto: Canadian Cancer Society; 2017. Available: http://www.cancer.ca/~/media/cancer.ca/CW/cancer%20information/cancer%20101/Canadian%20cancer%20statistics/Canadian-Cancer-Statistics-2017-EN.pdf (accessed 2018 February 27).
- Our investment. Edmonton: Alberta Cancer Foundation; 2018. Available: https://www.albertacancer.ca/our-investments (accessed 2018 Feb. 27).
- Cancer patient education, symptom management, mouth and dental care. Edmonton: Alberta Health Services; 2016. Available: https://www.albertahealthservices.ca/cancer/cancer.aspx (accessed 2018 Feb. 30).
- Cancer care Alberta. Edmonton: Alberta Health Services; 2018. Available: https://www.albertahealthservices.ca/cancer/cancer.aspx (accessed 2018 Feb. 30).
- Human papillomavirus (HPV-9) vaccine. Edmonton: Alberta Health Services; 2018. Available: https://www.albertahealthservices.ca/assets/info/hp/cdc/If-hp-cdc-hpv-info-sht-07-240-r01.pdf (accessed 2018 Feb. 30).
- Annual impact report: health 2015–16. Edmonton: Alberta Innovates; 2015-16. Available: https://albertainnovates.ca/wp-content/uploads/2018/02/Alberta-Innovates-Health-Impact-Report.pdf (accessed 2018 Feb. 30).
- About the ACPLF: Alberta Cancer Prevention Legacy Fund. Edmonton: Alberta Health Services; 2018. Available from: https://www.healthiertogether.ca/about/about-us/about-the-acplf/ (accessed 2018 Feb. 30).
- Project grant: fall 2018 results. Ottawa: Canadian Institutes of Health Research; rev. 2019. Available: https://cihr-irsc.gc.ca/e/51312.html (accessed 2019 Feb. 30).
- Gilbert R, Devries-Aboud M, Winquist E, Waldron J, McQuestion M, Head and Neck Cancer Disease Site Group. The management of head and neck cancer in Ontario.organizational and clinical practice guideline recommendations. Toronto: Cancer Care Ontario; 2013. Available: https://hncrehab.ca/wp-content/uploads/2015/06/Cancer-Care-Ontario-The-Management-of-head-and-neck-cancer-in-ontario.pdf (accessed 2021 Feb. 16).
- Human papillomavirus (hpv-9) vaccine. Government of Alberta. 2020. Available: https://myhealth.alberta.ca/Alberta/Pages/immunization-human-papillomavirus.aspx (accessed 23 Feb 2021)
- Ontario cancer facts: new cases of intra-oral cancers increasing after decades of decline. Toronto: Cancer Care Ontario; 2019. Available: https://www.cancercareontario.ca/en/cancer-facts/new-cases-intra-oral-cancers-increasing-after-decades-decline (accessed 2019 Feb. 30).
- Auluck A, Hislop G, Bajdik C, Poh C, Zhang L, Rosin M. Trends in oropharyngeal and oral cavity cancer incidence of human papillomavirus (HPV)‐related and HPV‐unrelated sites in a multicultural population: the British Columbia experience. Cancer. 2010;116(11):2635-44.
- Tota JE, Anderson WF, Coffey C, Califano J, Cozen W, Ferris RL, et al. Rising incidence of oral tongue cancer among white men and women in the United States, 1973–2012. Oral Oncol. 2017;67:146-52.
- Sankaranarayanan R, Ramadas K, Amarasinghe H, Subramanian S, Johnson N. Chapter 5: oral cancer: prevention, early detection, and treatment. In: Gelband H, Jha P, Sankaranarayanan R, Horton S, editors. Cancer: disease control priorities, 3rd ed., volume 3. Washington, DC: World Bank; 2015. Available: https://www.ncbi.nlm.nih.gov/books/NBK343649/ (accessed 2021 Feb. 16).
- Gupta N, Gupta R, Acharya AK, Patthi B, Goud V, Reddy S, et al. Changing trends in oral cancer — a global scenario Nepal J Epidemiol. 2016;6(4):613-9.
- Jaber MA. Dental practitioner’s knowledge, opinions and methods of management of oral premalignancy and malignancy. Saudi Dent J. 2011;23(1):29-36.
- Psoter WJ, Morse DE, Sánchez-Ayendez M, Vega CMV, Aguilar ML, Buxó-Martinez CJ, et al. Increasing opportunistic oral cancer screening examinations: findings from focus groups with general dentists in Puerto Rico. J Cancer Educ. 2015;30(2):277-83.





