Abstract
Objectives:
The aims of this study were to determine demographic profiles, tumour characteristics and treatment factors related to oral cavity and oropharyngeal cancer (OCC and OPC) and comparatively analyze these cancers in the adult population of Alberta, Canada, over 12 years.
Methods:
Demographic, tumour characteristics and treatment data regarding OCC and OPC incidence in Alberta residents ≥18 years in 2005–2017 were extracted from the Alberta Cancer Registry database. Age-standardized incidence and mortality rates (ASIR and ASMR) were computed.
Results:
Among 3448 OCC and OPC cases, mean (standard deviation) age at diagnosis was 63.9 (14.4) and 60.1 (10.2) years, respectively. There was a male predilection for both OCC (58.2%) and OPC (81.7%). With some fluctuations, ASIR remained the same for OCC but increased for OPC. ASMR increased for both. The most common site for OCC was tongue and for OPC tonsil. Squamous cell carcinoma was the most common diagnosis for OCC and OPC. Involvement of at least 1 lymph node was observed in 38.5% of OCC and 85.8% of OPC cases. For 45.2% of OCC and 82.3% of OPC cases, diagnosis occurred at stage IV. The most common initial treatments for OCC were surgery, alone or combined with radiation, whereas radiation with chemotherapy was the main treatment modality for OPC.
Conclusions:
The incidence of OPC in younger males was higher than that of OCC. Although incidence of OPC per 100 000 population increased over the 12-year study period, it remained largely unchanged for OCC. For both cancers, initial diagnoses were made at advanced stages, with almost twice as many stage IV OPC cases than OCC cases.
Oral cavity cancer (OCC) and oropharyngeal cancer (OPC) remain a major public health concern worldwide because of the significant associated morbidity and mortality rates.1 OCC and OPC are grouped within head and neck squamous cell carcinoma, which is the sixth most common malignancy in the world.2 According to International Agency for Research on Cancer data, approximately 440 000 new cases and 220 000 deaths would occur globally in 2018 from OCC and OPC.3 In 2020, an estimated 5400 Canadians would be diagnosed with oral cancer and 1500 associated deaths would occur.4 Despite technological advances in diagnosis and treatment, the global 5-year survival rate remains at approximately 50%,5 perhaps because of delays in diagnosis and treatment related to both the patient and the health care system.6
Although the worldwide incidence of OCC has been decreasing over the past 30 years because of a decline in tobacco use, the incidence of OPC has been increasing in the past few decades.7 This increase in OPC has been noted particularly in Western countries, including the United States and Canada.8 There is a strong association between OPC and sexually transmitted human papilloma virus (HPV) infection.7 Although both OCC and OPC show a strong male predilection and are mainly diagnosed as squamous cell carcinoma microscopically, there are other noteworthy distinctions between these entities. Clinically, OPC occurs in a younger age group compared with OCC, and patients are generally white, healthier, non-smokers and have higher socioeconomic status.9 Furthermore, OPC is site specific to the oropharynx, particularly the tonsil and base of tongue,10,11 whereas the tongue is the most common location for OCC.2 Although patients with OPC present with early-stage primary tumours (T1 or T2), they commonly have nodal involvement at the time of diagnosis,12 which indicates advanced disease. OPC is associated with better treatment outcomes and higher survival rate despite initially presenting with nodal disease.12,13 OPC treatment generally consists of radiation and/or chemotherapy rather than surgery, whereas surgery with or without radiation is used in OCC.14
Although many studies have been conducted on OCC and OPC globally, little has been reported on their changing trends in Canada. Therefore, the objectives of this study were to create a detailed clinicopathologic and demographic profile of patients diagnosed with OCC and OPC in Alberta, Canada, and to carry out a comparative analysis between the 2 types of cancer in a 12-year period.
Materials and Methods
Data Collection
Data were extracted from the Alberta Cancer Registry (ACR) database after obtaining ethics approval from the Health Research Board of Alberta Cancer Committee (HREBA.CC-17-0370). The ACR is a population-based registry, operated by Alberta Health Services, that records information on new cancer cases in Alberta. Data were obtained for Alberta residents who were diagnosed with primary oral cavity and/or oropharyngeal malignancies at age ≥18 years between 2005 and 2017.
Tumour sites were categorized according to the topographical codes in the International Classification of Diseases for Oncology, 3rd edition (ICD-O-3). OCC sites included lip mucosa (C00.3–C00.9), oral tongue (C02.0–C02.3, C02.8 and C02.9), gum (C03.0–C03.9), floor of mouth (C04.0–C04.9), palate (C05.0–C05.9) and other and unspecified parts of the mouth (C06.0–C06.9). OPC sites included base of tongue (C01.9), lingual tonsil (C02.4), tonsil (C09.0–C09.9), oropharynx (C10.0–C10.9), pharynx not otherwise specified (NOS) (C14.0) and Waldeyer ring (C14.2). Excluded sites were external upper and lower lip (C00.0–C00.1), parotid gland (C07.9) and other unspecified major salivary gland tumours (C08.0–C08.9). Selection of these anatomic sites specific to OCC and OPC was based on updated criteria presented at the 2018 American Society of Clinical Oncology Annual Meeting.15
Demographic factors included gender, age at diagnosis, year of diagnosis, age at death, vital status, urban vs. rural geographic zone at diagnosis and household income. Income data were based on neighbourhood and were obtained from Statistics Canada 2006 Census. Tumour-related factors included tumour morphology description/tumour histologic diagnosis and histologic grading according to ICD-O-3, tumour stage as reported in pathology reports according to the American Joint Committee on Cancer (AJCC), 6th and 7th editions. Treatment modality factors included surgery, chemotherapy, radiation, immunotherapy and combinations of treatment.
Statistical Analysis
Age-standardized incidence (ASIR) and age-standardized mortality rate (ASMR) were computed. To calculate ASIR, a weighted average of the number of new OCC and OPC cases per 100 000 people in a 5-year age group (0–4, 5–9, etc., to ≥85 years) diagnosed each year between 2005 and 2017 was divided by the total number of Albertans in that age group that year. A similar method was followed for ASMR using the number of deaths from OCC and OPC instead of the number of new cases. The 2011 Canadian standard population was used in both analyses for measuring weighted averages.
Various aspects of all available numerical and categorical data were described for and compared between OCC and OPC. Any possible difference between the 2 cancer groups for categorical variables was examined using Pearson’s Χ2 or Fisher’s exact test. Numerical variables were examined using Student’s t test. Statistically significant results were reported at p < 0.05 for 2-sided tests. SAS v. 9.2 was used for all statistical analyses.
Results
Demographic Characteristics
A total of 3448 OCC and OPC patients were identified from the Alberta Cancer Registry database from 2005 to 2017. Of these, 1763 (51.1%) represented OCC and 1685 (48.9 %) were diagnosed with OPC. There was a stronger male predilection among OPC cases (81.7%) compared with OCC (58.2%) (p < 0.001). The mean (standard deviation) age at diagnosis was 63.9 (14.3) years for OCC and 60.1 (10.2) years for OPC. More OPC cases were identified among the 46–65 years age group than other age groups (p < 0.001). A larger proportion of people with OCC (64.1%) were >65 at time of death than people with OPC (36.0%) (p < 0.001) (Table 1). Cause of death was not included because it was not consistently specified for every patient in the cancer registry. About 3 quarters of patients with OCC and OPC (74.6% and 75.6%, respectively) had an annual household income of ≥$45 000. Higher numbers of OCC and OPC cases were diagnosed in urban centres compared with rural areas; however, the difference was not statistically significant (p = 0.888) (Table 1). Over the 12-year study period, overall ASIR remained the same for OCC, but increased for OPC (p = 0.004, MannKendall trend test) (Figure 1). Changes in mortality rate, however, were not statistically significant for either OCC or OPC.
|
Characteristic |
OCC, no. (%) |
OPC, no. (%) |
p* |
|---|---|---|---|
|
Note: SD = standard deviation. |
|||
| Gender Male Female |
1026 (58.2) 737 (41.8) |
1377 (81.7) 308 (18.3) |
< 0.001 |
| Mean age, years (SD) | 63.9 (14.3) | 60.1 (10.2) | |
| Age group, years < 45 46–65 > 65 |
166 (9.4) 798 (45.3) 799 (45.3) |
98 (5.8) 1139 (67.6) 448 (26.6) |
< 0.001 |
| Annual income, $ < 45K 45–75K > 75K |
447 (25.4) 779 (44.2) 537 (30.5) |
411 (24.4) 664 (39.4) 610 (36.2) |
0.001 |
| Geographic area Urban Rural |
1355 (76.9) 408 (23.1) |
1300 (77.1) 387 (23.0) |
0.888 |
| Characteristic |
OCC, no. (%) |
OPC, no. (%) |
p* |
| Age at death, years ≤ 45 46-65 > 65 |
25 (2.6) 278 (29.4) 642 (67.9) |
9 (1.3) 319 (45.1) 379 (53.6) |
< 0.001 |
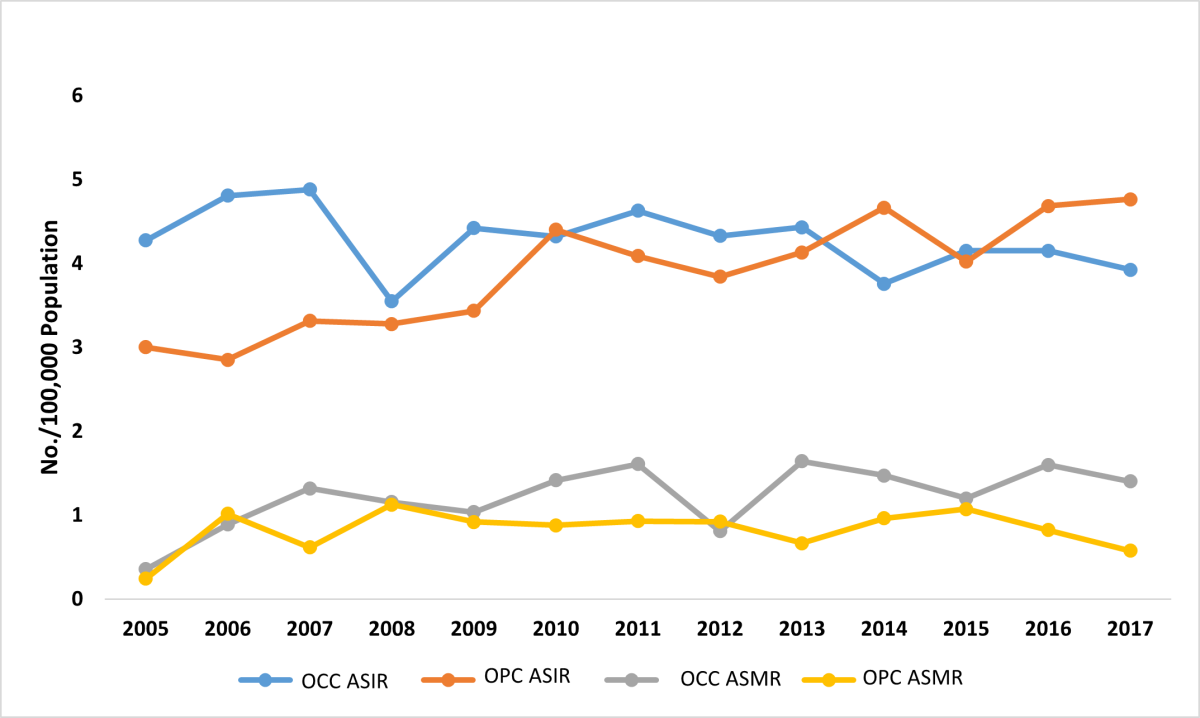
Figure 1: Age-standardized incidence (ASIR) and mortality rate (ASMR) for oral cavity cancer (OCC) and oropharyngeal cancer (OPC) in Alberta patients, 2005–2017.
Tumour-Related Characteristics
The most common primary site for OCC was tongue with 43.6% of cases (24.8% in males, 18.8% in females), followed by mouth, other and unspecified (17.0%: 9.8% in males and 7.2% in females) and floor of the mouth (15.7%: 10.4% in males and 5.3% in females) (p < 0.001) (Figure 2). The most common primary site for OPC was tonsil with 48.3% of cases (38.9% in males, 9.4% in females), followed by base of tongue (38.1%: 32.4% in males and 5.7% in females) and oropharynx (12.1%: 9.1% in males and 3.0% in females) (p = 0.019) (Figure 3). There was a predominance of squamous cell carcinoma, which was diagnosed in 97.9% of OCC and 94.0% of OPC cases, respectively. Regarding histologic grade, 41.4% of the malignancies were moderately differentiated, moderately-to-well differentiated or of intermediate differentiation, followed by 24.7% poorly differentiated tumours and 20.8% where differentiation was not determined, not stated or not applicable; the remainder were well differentiated/differentiated NOS (11.8%) or undifferentiated/differentiated NOS tumours (1.5%).
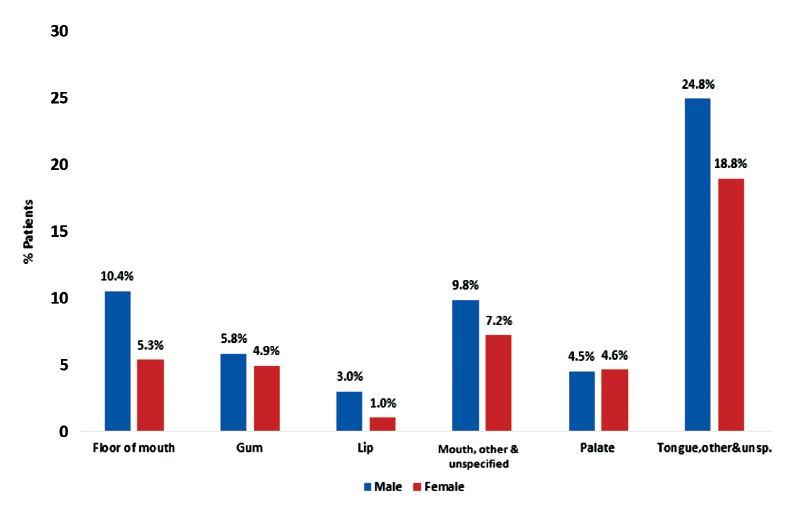
Figure 2: Distribution of oral cavity cancer (OCC) in Alberta patients, 2005–2017, by anatomic subcategories (Χ2 test, p < 0.001).
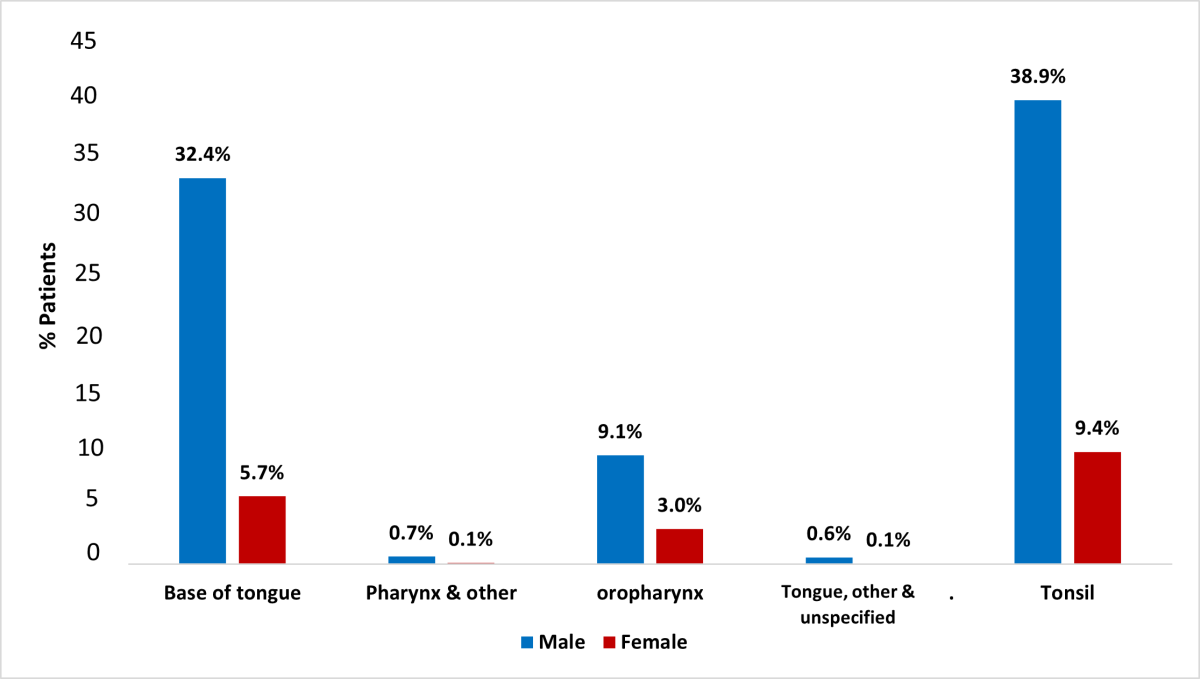
Figure 3: Distribution of oropharyngeal cancer (OPC) in Alberta patients, 2005–2017, by anatomic subcategories (Fisher’s exact test, p = 0.019).
Based on the TNM (tumour, nodes, metastases) staging system, smaller proportions of tumours were at stages T0 and T3 than other stages (Figure 4) for both OCC and OPC groups (p < 0.001). Most OPC patients (85.8%) had at least 1 clinically apparent lymph node at the time of diagnosis with the highest frequency associated with tonsil tumours (48.3%); only 38.5% of OCC patients presented with lymph node involvement (p < 0.001). Higher nodal involvement occurred with tongue malignancies (45.9%) compared with other OCC sites. Few cases of distant metastases were found at the time of diagnosis (OCC 1.9%, OPC 4.7%, p < 0.001). Most OCC and OPC cases were diagnosed at an advanced clinical stage, with significantly higher numbers of stage IV OPC: 82.3% of OPC cases versus 45.2% of OCC cases (p < 0.001) (Figure 5). In the OPC group, more tumours of the tonsil presented at stage IV (46.5%) than other sites (p < 0.001). Among OCC patients, more tumours of the tongue (37.6%) presented at stage IV than other sites; most lip tumours were diagnosed at stage I (56.3%) (p < 0.001).
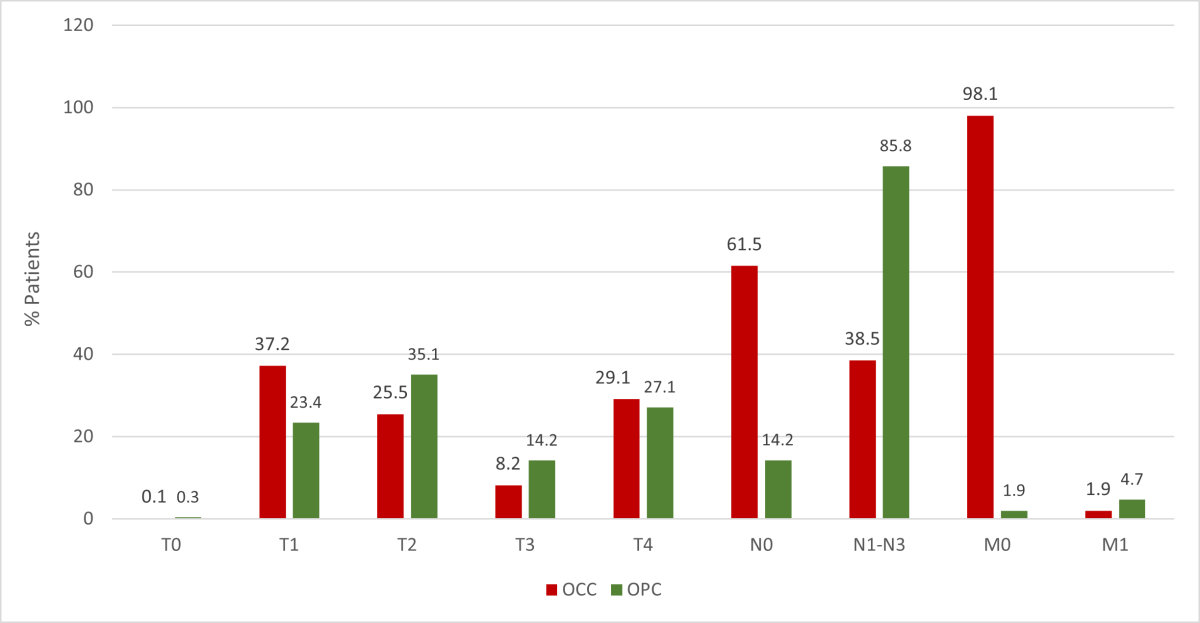
Figure 4: Distribution of oral cavity cancer (OCC) and oropharyngeal cancer (OPC) in Alberta patients, 2005–2017, according to TNM staging (p determined by Χ2 test for T, N and M).
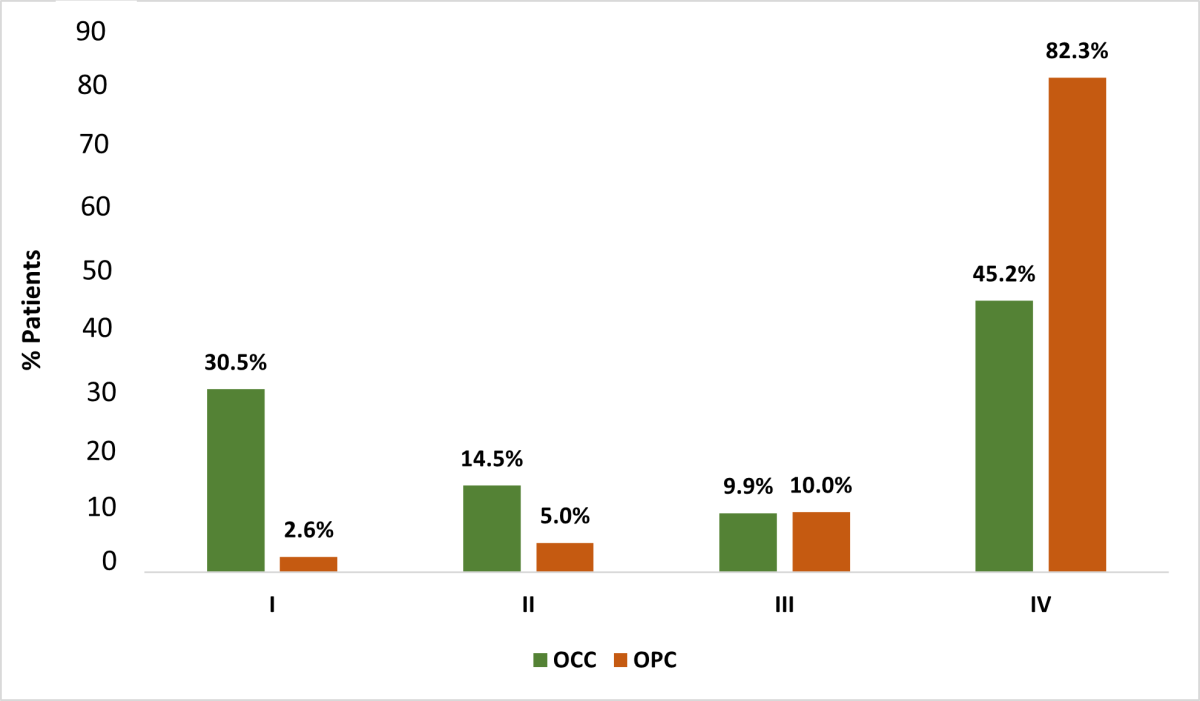
Figure 5: Distribution of oral cavity cancer (OCC) and oropharyngeal cancer (OPC) in Alberta patients, 2005–2017, according to clinical stage (I–IV) based on American Joint Committee on Cancer, 6th and 7th editions (Χ2 test, p < 0.001).
Treatment Factors
Of 3448 OCC and OPC patients, 313 (9.1%) patients did not receive any initial treatment at diagnosis, and the reasons for this are unknown. More OCC patients (50.9%) received surgical treatment only compared with 8.5% of OPC patients (p < 0.001) (Table 2). Conversely, 40.3% of OPC patients received radiation and chemotherapy combined compared with 2.6% of OCC patients (p < 0.001).
|
Treatment |
OCC, % |
OPC, % |
p |
|---|---|---|---|
|
Note: N/A = not applicable. |
|||
| Surgery only | 50.9 | 8.5 | < 0.001* |
| Radiation only | 7.3 | 11.3 | < 0.001* |
| Chemo only | 0.2 | 0.4 | 0.540† |
| Surgery + radiation | 20.0 | 9.9 | < 0.001* |
| Radiation + chemo | 2.6 | 40.3 | < 0.001* |
| Surgery + radiation + chemo | 8.8 | 17.3 | < 0.001* |
| Other combinations | 0.3 | 4.0 | N/A |
| None | 9.9 | 8.3 | 0.098* |
Discussion
This is the first known study to investigate and compare OCC and OPC cancers in Canada in terms of demographic, tumour and treatment factors.
For all OPC sites (base of tongue, tonsil and oropharynx), there was a strong male predilection. This tendency has been reported for OPC in the United States and globally.7,11 For OCC, male to female ratios were close to 1 for all sites except floor of mouth and lip, where tumours were more prevalent in males. Although the average age for combined OCC and OPC patients was 62.3 years (OCC 63.9 years, OPC 60.1 years), a younger average age at diagnosis was noted for OPC patients with base of tongue (60.7 years) and tonsil (58.9 years) tumours. This correlates with other studies showing OPC occurring at a younger age than OCC.9
In our study, there was a slight difference in rates of OCC and OPC associated with income. Patients with HPV-positive OPC have been reported to have higher household income and education than patients with HPV-negative OPC, possibly because of liberal sexual behaviour.16 Other studies have also shown lower disease-specific and overall survival for all head and neck cancer locations/subsites in neighbourhoods with low socioeconomic status.17 However, only a few have shown a direct association with socioeconomic status, perhaps because of the multifactorial nature of head and neck cancer. Considerations include stage at diagnosis, education, access to health care and tobacco and alcohol use.18
The incidence of OCC generally remained the same between 2005 and 2017 and rose for OPC during this period. These findings, although inconsistent with notably decreasing rates of OCC globally and in the United States,7 are similar to those of a New Zealand study, which showed incidence trends for OCC rising slightly at the end of the 30-year study period, but with no statistical significance.19 One possible explanation for the lack of notable change or decrease in incidence of OCC cases in this study is that the growing economy over the past 2 decades has resulted in a significant increase in the population of Alberta, from 2.9 million in 2000 to 4.4 million in 2020.20 Another factor may be associated with the ethnic origin of a portion of those moving to Alberta. Statistics Canada showed increased migration of people from South and Southeast Asia between 2011 and 2016 to the larger provinces including Alberta. In Alberta, the percentage of recent immigrants rose from 9.3% in 2006 to 17.1% in 2016.21 People from these regions have been shown to have a predisposition for head and neck cancer, likely associated with tobacco and betel nut use.22 This trend, of a predisposition of head and neck cancer in those from South and Southeast Asia related to tobacco and betel nut use, has been reported in some European countries with high numbers of South Asian immigrants.23,24
As in other studies, the most common location for OCC was tongue and for OPC tonsil.25,26 The most common histologic diagnosis was squamous cell carcinoma NOS, but keratinizing NOS and nonkeratinizing large-cell NOS variants were also seen. With the recognition of HPV-associated carcinomas of the oropharynx as distinct tumours in the early 2000s, the WHO 2005 classification of tumours placed both conventional OCC and OPC in the same category and both were morphologically classified as well, moderately and poorly differentiated groups.27 In 2014, several suggestions for the next WHO classification of head and neck tumours were proposed with regard to HPV-associated OPC.28 “Nonkeratinizing” squamous cell carcinoma was proposed for this subset of HPV-positive tumours demonstrating favourable outcomes. In our series, it is possible that more keratinizing NOS and nonkeratinizing large-cell NOS carcinomas were diagnosed and included in the registry after acceptance of the proposed terminology.
Individual stage component findings for T, N and M and overall stage (AJCC 6 and 7) for OCC and OPC were similar to those reported previously.25,29 Significantly higher numbers of OPC cases than OCC presented at stage IV, and this is similar to other reported studies.30 It is well known that HPV-associated OPC has a better overall post-treatment outcome including survival compared to OCC.13,14 In 2016, a revised staging classification system, AJCC 8, was developed to reflect the unique characteristics of HPV-associated OPC, as earlier versions were based on HPV-negative cancers related to traditional risk factors including smoking. An HPV-positive OPC case staged as T1N2M0 (2-cm tumour with 2 involved lymph nodes) and overall stage IV in AJCC 7 is now considered stage I in the AJCC 8 because the prognosis is similar to that for curable cancers.10,15 Because the staging information available in the Alberta Cancer Registry included only AJCC 6 and 7 until 2017, it is possible that a number of stage IV OPC tumours would now be considered a lower stage under the new classification. The lack of tumour HPV status in this registry will likely hinder classifying these tumours under AJCC 8.
Despite the existence of publicly funded health care in Alberta, significant numbers of OCC and OPC cases were diagnosed at a late stage. Possible reasons include lack of awareness about these cancers, delay in seeking care, long wait times to see specialists and denial or fear of an existing problem.6 Detection of oropharyngeal lesions is difficult, because of limited visibility of anatomic sites beyond the oral cavity and lack of symptoms until the disease has progressed. Thus, screening is challenging compared with cancers of the oral cavity, which is easier to examine. Treatment modalities for OCC and OPC patients were similar to those offered at other institutions. Clearly, radiation or radiation/chemotherapy treatment for OPC is aimed at preserving function and reducing morbidity. As demonstrated by several studies, the general outcome for OPC has been favourable with various treatment modalities.13,14
Some limitations of this study include lack of data in the Alberta Cancer Registry regarding HPV status, smoking and alcohol history and ethnicity. Furthermore, indication of social status, including education and family income at an individual level could be more representative than neighbourhood socioeconomic status, on which our findings are based.
Future studies directed at investigating risk factors, including tobacco and alcohol consumption and ethnic origin in this study group may explain why the incidence of OCC stayed the same over the 12-year study period. Determining HPV status of OPC cases in this study group and comparing it with other jurisdictions in Canada, North America and the world could contribute to the widening body of research on this important topic.
Conclusions
The results of this study confirm a number of findings of other descriptive studies of OCC and OPC, including gender predilection, age at diagnosis and death, anatomic site, histologic diagnosis, clinical stage and treatment. They also support the need for early detection through screening examinations, increased awareness of oral/oropharyngeal cancer and earlier access to care. A survival analysis to achieve better understanding of post-diagnosis and post-treatment life span among these groups of patients is recommended.
THE AUTHORS
Corresponding author: Dr. Seema Ganatra, Clinical Professor, Oral Medicine and Pathology, 5-395 ECHA 11405 87 Avenue NW, Edmonton AB T6G 1C9. Email: sganatra@ualberta.ca
The authors have no declared financial interests.
This article has been peer reviewed.
Availability of data and material: Data from the Alberta Cancer Registry (ACR) database were made available once ethics approval was obtained from the Health Research Board of Alberta Cancer Committee. The ACR is a population-based registry operated by Alberta Health Services. Our team worked closely with a data analyst (surveillance and reporting) at C-MORE/CancerControl Alberta/Alberta Health Services.
References
- Oskam IM, Verdonck-de Leeuw IM, Aaronson NK, Witte BI, de Bree R, Doornaert P, et al. Prospective evaluation of health-related quality of life in long-term oral and oropharyngeal cancer survivors and the perceived need for supportive care. Oral Oncol. 2013;49(5):443-8.
- Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009;45(4-5):309-16.
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394-424.
- Brenner DR, Weir HK, Demers AA, Ellison LF, Louzado C, Shaw A, et al. Projected estimates of cancer in Canada in 2020. CMAJ. 2020;192(9) E199-205.
- Shield KD, Ferlay J, Jemal A, Sankaranarayanan R, Chaturvedi AK, Bray F, et al. The global incidence of lip, oral cavity, and pharyngeal cancers by subsite in 2012. CA Cancer J Clin. 2017; 67(1):51‐64.
- Stefanuto P, Doucet JC, Robertson C. Delays in treatment of oral cancer: a review of the current literature. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014; 117(4):424-9.
- Javadi,P, Sharma A, Zahnd WE, Jenkins WD. Evolving disparities in the epidemiology of oral cavity and oropharyngeal cancers. Cancer Causes Control. 2017;28(6):635-45.
- de Martel C, Plummer M, Vignat J, Franceschi S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int J Cancer. 2017;141(4):664-70.
- Viens LJ, Henley SJ, Watson M, Markowitz LE, Thomas CC, Thompson TD, et al. Human papillomavirus-associated cancers — United States, 2008–2012. MMWR Morb Mortal Wkly Rep. 2016;65(26):661-6.
- Tanaka TI, Alawi F. Human papillomavirus and oropharyngeal cancer. Dent Clin N Am. 2018;62(1);111-20.
- Chaturvedi AK, Anderson WF, Lortet-Tieulent J, Curado MP, Ferlay J, Franceschi S, et al. Worldwide trends in incidence rates for oral cavity and oropharyngeal cancers. J Clin Oncol. 2013;31(36);4550-9.
- O’Sullivan B, Huang SH, Su J, Garden AS, Sturgis EM, Dahlstrom K, et al. Development and validation of a staging system for HPV-related oropharyngeal cancer by the International Collaboration on Oropharyngeal cancer Network for Staging (ICON-S): a multicentre cohort study. Lancet Oncol. 2016;17(4):440-51.
- Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tân PF, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24-35.
- Wang MB, Liu IY, Gornbein JA, Nguyen CT. HPV-positive oropharyngeal carcinoma: a systematic review of treatment and prognosis. Otolaryngol Head Neck Surg. 2015 Nov;153(5):758-69.
- Lydiatt WM, Patel SG, O’Sullivan B, Brandwein MS, Ridge JA, Migliacci JC, et al. Head and neck cancers — major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017; 67(2):122-37.
- Dahlstrom KR, Bell D, Hanby D, Li G, Wang LE, Wei Q, et al. Socioeconomic characteristics of patients with oropharyngeal carcinoma according to tumor HPV status, patient smoking status and sexual behavior. Oral Oncol. 2015;51(9):832-8.
- Andersen ZJ, Lassen CF, Clemmensen IH. Social inequality and incidence of and survival from cancers of the mouth, pharynx and larynx in a population-based study in Denmark, 1994–2003. Eur J Cancer. 2008;44(14):1950-61.
- McDonald JT, Johansen-Obaseki S, Hwang E, Connell C, Corsten M. The relationship between survival and socioeconomic status for head and neck cancer in Canada. J Otolaryngol Head Neck Surg. 2014;43(1):2.
- Chelimo C, Elwood JM. Sociodemographic differences in the incidence of oropharyngeal and oral cavity squamous cell cancers in New Zealand. Aust N Z J Public Health. 2015;39(2):162-7.
- Population: estimates of the number of people living in Canada and the provinces. Edmonton: Government of Alberta; 2020. Available: https://economicdashboard.alberta.ca/Population (accessed 2021 June 4).
- Focus on geography series, 2016 Census: immigration and ethnocultural diversity. Ottawa: Statistics Canada; 2017. Available: https://www12.statcan.gc.ca/census-recensement/2016/as-sa/fogs-spg/Facts-can-eng.cfm?Lang=Eng&GK=CAN&GC=01&TOPIC=7 (accessed 2021 June 4).
- Petti S, Masood M, Scully C. The magnitude of tobacco smoking-betel quid chewing-alcohol drinking interaction effect on oral cancer in South-East Asia: a meta-analysis of observational studies. PLoS One. 2013;8(11):e78999.
- Warnakulasuriya KA, Johnson NW, Linklater KM, Bell J. Cancer of mouth, pharynx and nasopharynx in Asian and Chinese immigrants resident in Thames region. Oral Oncol. 1999;35(5):471-5.
- Mangtani P, Maringe C, Rachet B, Coleman MP, dos Santos Silva I. Cancer mortality in South Asian migrants in England and Wales (1993–2003): patterns in the overall population and in first and subsequent generations. Br J Cancer. 2010;102(9):1438-43.
- Jéhannin-Ligier K, Dejardin O, Lapôtre-Ledoux B, Bara S, Coureau G, Grosclaude P, et al. Oral cancer characteristics in France: descriptive epidemiology for early detection. J Stomatol Oral Maxillofac Surg. 2017;118(2):84-9.
- Chaturvedi AK, Engels EA, Pfeiffer RM, Hernandez BY, Xiao W, Kim E, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29(32):4294-301.
- Barnes L, Eveson JW, Reichart P, Sidransky D, editors. Pathology and genetics: head and neck tumours. In World Health Organization classification of tumours, 3rd ed., volume 9. Lyon: International Agency for Research on Canacer; 2005. 430 p.
- Lewis Jr JS. Chernock RD. Human papillomavirus and Epstein Barr virus in head and neck carcinomas: suggestions for the new WHO classification. Head Neck Pathol. 2014;8(1):50-8.
- Chernock RD, El-Mofty SK, Thorstad WL, Parvin CA, Lewis Jr JS. HPV-related nonkeratinizing squamous cell carcinoma of the oropharynx: utility of microscopic features in predicting patient outcome. Head Neck Pathol. 2009;3(3):186-94.
- Carrillo JF, Carrillo LC, Cano A, Ramirez-Ortega MC, Chanona JG, Aviles A, et al. Retrospective cohort study of prognostic factors in patients with oral cavity and oropharyngeal squamous cell carcinoma. Head Neck. 2016;38(4):536-41.





