View letters related to this article
Abstract
A primary molar dental abscess was implicated as the cause of a brain abscess in an 11-year-old boy. This case report describes the neurological signs and symptoms, and acute management of a brain abscess in a child. A brain abscess is provisionally diagnosed from the patient’s medical history, as well as the presence of signs and symptoms such as fever, headache, nausea, vomiting, focal neurological deficit, altered mentation, speech alterations, papillary edema, and neck stiffness or seizures. A definitive diagnosis of brain abscess is confirmed through imaging. The dental source of infection is identified by the exclusion of more probable foci such as the ears, heart, lungs, eyes or sinuses.
Introduction
Dental abscesses and facial cellulitis put dentists on alert for potentially life-threatening conditions such as sepsis or airway obstruction, but the risk of a brain abscess is a complication of odontogenic infection that dentists rarely consider. This report describes the case of an 11-year-old boy whose brain abscess and associated neurological signs were most likely attributable to an abscessed primary molar (Figs. 1a and 1b). The description of the neurological signs and symptoms, and the history and management of this case will inform dentists about the real possibility of a brain abscess of dental origin.
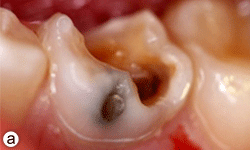 Figure 1a: Grossly decayed primary mandibular left second molar in an 11-year-old boy.
Figure 1a: Grossly decayed primary mandibular left second molar in an 11-year-old boy.
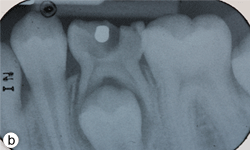 Figure 1b: Periapical radiograph displaying gross decay and furcation radiolucency in tooth 75.
Figure 1b: Periapical radiograph displaying gross decay and furcation radiolucency in tooth 75.
Brain abscesses are rare, but serious, life-threatening conditions associated with high morbidity and mortality.1-6 A brain abscess is a focal infection in the parenchyma of the brain, characterized by local edema and inflammation that develops into a well-circumscribed collection of pus.7 Brain abscesses result from the direct extension of a contiguous suppurative focus, from hematogenous dissemination from a distant focus, or from direct inoculation during a trauma or neurosurgery.1,8 However, in up to 15%–30% of cases, the cause of brain abscesses is unknown.9 The majority of brain abscesses arise from the direct spread of infection through the facial planes, and often originate in the paranasal sinuses, middle ear and mastoid area.1,10 Brain abscesses are rare in children and are most likely to occur within the first decade of life because of the higher prevalence of sinus and middle ear infections in this age group.1,3,7,11 Congenital heart disease, sinus infections, ear infections and a compromised immune system are the most common predisposing factors in children diagnosed with brain abscess.1,3,7,11 When the spread of infection is blood-borne, its pathway is through general blood circulation or by blood flow through the facial and ophthalmic veins where pathogens enter the cranium through the cavernous sinus.10,12-14 Zhang and Stringer,10 who dissected multiple valves in the facial and ophthalmic veins previously believed to be valveless, attributed the increased risk of hematogenous spread of infection from the mid face to consistent communication between the facial vein, pterygoid plexus, and angular and ophthalmic veins, and the cavernous sinus, rather than to retrograde blood flow through valveless veins. The most common primary sources of brain abscesses are infective endocarditis, osteomyelitis, bacteremia, and pulmonary, abdominal, pelvic, skin or dental infections.9
Fever, headache, nausea, vomiting, focal neurological deficit, altered mentation, altered speech, lethargy, papillary edema, neck stiffness, and increased intracranial pressure or seizures are common clinical presentations of a brain abscess.3,15,16 Signs and symptoms of neurological deficit depend on the area of the brain affected.
Because brain abscesses are rare, potential primary sources of infection are multiple and the bacteria present in the oral cavity are diverse, a diagnosis of a brain abscess caused by a dental infection is made by exclusion. Criteria used to implicate a dental source of infection for a brain abscess include finding no other source of infection, oral microflora in the microbiological spectrum of the brain abscess, and clinical and radiographic signs of an acute or chronic dental infection.17
Dentists should understand that even a quiescent nonvital tooth could produce blood-borne bacterial sepsis. The presence of aberrant neurological signs and symptoms may indicate the early stages of a brain abscess. A physician’s diagnosis of a brain abscess is followed by the search for the primary source of infection. Identification of a dental focus of infection is essential for both diagnosis and treatment.
Case Report
An 11-year-old previously healthy boy came to a community hospital emergency department with a 2-week history of a dull continuous headache and 1-week history of nausea and vomiting. He was discharged with a differential diagnosis of influenza or migraines. Bed rest and supportive treatment were recommended.
The following day, the patient was rushed to the emergency department at Timmins and District Hospital after his mother found him lying on the floor screaming, holding his head, mumbling and unable to speak in words (dysphasia). He displayed an acute onset of confusion, lethargy, unstable gait and neck stiffness. Magnetic resonance imaging investigations (Figs. 2a and 2b) revealed a 4.7 × 4.4 × 3.4 cm thin-walled lesion with surrounding edema in the left temporal lobe. The patient was started on a regimen of dexamethasone, mannitol, vancomycin and ceftriaxone, and was immediately airlifted to The Hospital for Sick Children (SickKids) in Toronto.
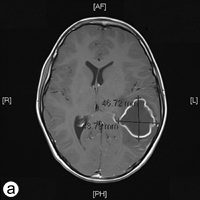
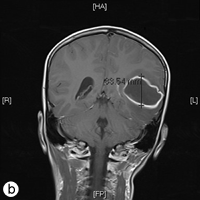
Figure 2: a) Axial and b) coronal views of the magnetic resonance imaging reveal a 4.7 × 4.4 × 3.5 cm abscess in the left temporal lobe of the brain.
Upon arrival in the emergency department at SickKids (day 1), the patient was awake, alert and oriented to time and place, and had full range of motion of his limbs. However, he had pronounced dysphasia. His medical history revealed no recent sinusitis, otitis media or upper respiratory tract infections, but he did report having a toothache about 3 weeks before that had since subsided. The patient’s mother reported a history of swelling that was localized to the tooth 2 weeks before that had subsequently ruptured and the associated pain had subsided. The patient was admitted to neurosurgery (day 2) and underwent a craniotomy and ultrasound-guided aspiration of a temporoparietal intracerebral abscess on the left-side of the head without complication. Culture and sensitivity tests were done, and he continued with a regimen of broad-spectrum intravenous antibiotics that included vancomycin, ceftriaxone and metronidazole. The next day the patient reported having what he called a “funny feeling” and had a hemifacial droop on the right that was highly suggestive of a partial focal seizure. As a result, he was prescribed phenytoin. A computed tomography scan demonstrated a decrease in the size of the abscess, a persistent mass-effect midline shift to the right and diffuse cerebral edema.
Dental examination revealed gross decay of the lower left second primary molar (tooth 75). No swelling, purulence or other signs of infection were noted. Intraoral radiographs showed evidence of a furcation radiolucency associated with tooth 75 (Figs. 1a and 1b). A diagnosis of dental abscess was made based upon clinical signs and radiographic findings. Subsequently, tooth 75 was extracted under general anesthesia (day 5); cultures were collected and sent for culture and sensitivity.
One week later (day 14), follow-up imaging demonstrated a recurrence of the temporoparietal abscess on the left. A second craniotomy was done, and aspirates were again collected for microbiological analysis.
Microbiological results yielded the following findings. Streptococcus anginosus was isolated from the cultures obtained during the first craniotomy procedure (day 2). Cultures from the site of the dental extraction produced numerous Streptococcus species with heavy growth under aerobic conditions and heavy growth of the usual flora with few neutrophils under anaerobic conditions. The second craniotomy culture contained many neutrophils, but no microflora were cultured.
Significant neurological improvement was noted after the second craniotomy. Regular follow-ups included weekly computed tomography imaging. Images demonstrated an ongoing reduction in the size of the abscess. Overall neurological function improved, but the patient continued to have difficulty with comprehension.
Five weeks after his initial presentation, the patient was transferred to Holland Bloorview Kids Rehabilitation Hospital (formerly Bloorview Kids Rehab), a pediatric rehabilitation hospital, for intensive rehabilitation therapy. Ninety-three days after his initial presentation to SickKids, the patient was discharged home. He continues rehabilitation under the care of speech and language pathologists and occupational therapists at Bloorview on an outpatient basis. He was prescribed levetiracetam and was on a 6-month follow-up schedule with the neurosurgery department at SickKids.
Discussion
Dental infections have been implicated as the cause in multiple cases of brain abscesses and the recent death of a child in Boston.8,18,19 Reported dental sources of brain abscesses have included dental abscess, cellulitis, periodontitis, extractions, root-canal therapy, periodontitis, application of braces and osteomyelitis.8,18,20 Microorganisms that are abundant in the oral mucosa and dental plaque such as gram-positive bacteria (e.g., Streptococcus and Staphylococcus), gram-negative bacteria (e.g., Haemophilus) and fungi are commonly cultured from brain abscesses, implicating the oral cavity as a probable primary source of infection.1,3,21,22
The intracerebral abscess reported in this case was likely the result of a dental infection. Diagnosis was made by exclusion of other sources of infection. The criteria applied that implicate a dental source of infection as the cause of a brain abscess in this case are the following17:
- No other source of infection is found: The current patient’s medical history and clinical investigations ruled out recent sinusitis, otitis media or upper respiratory infection as possible contributors. An echocardiogram revealed no evidence of intracardiac shunts or vegetations and demonstrated laminar flow in the inferior vena cava, superior vena cava and abdominal aorta. Chest and abdominal radiographs provided no significant findings.
- The microbiological spectrum should contain the oral microflora: Streptococcus anginosus was isolated from the specimen collected during this patient’s first craniotomy. S. anginosus is commonly found in saliva, dental plaque, dental infections and the gastrointestinal and genitourinary tracts.23 S. anginosus has been strongly associated with purulent infections, bacteremia and intracerebral abscesses because of its high resistance to phagocytosis by human polymorphonuclear leukocytes.21,24 S. anginosus from the oral cavity likely gained entrance to the vascular system and spread to the parenchyma of the brain.
- Clinical and radiographic signs and symptoms of an acute or chronic dental infection are present: This patient’s dental history, and clinical and radiographic examinations revealed a dental abscess of the lower left second primary molar (tooth 75). Clinically, decay was noted and evidence of a furcation radiolucency associated with tooth 75 was present on the radiograph.
Recurrence of a brain abscess is not uncommon (6%–87%); the rate of recurrence depends on the location and size of the abscess and the choice of treatment.25 The majority of recurrences occur within 2 weeks of treatment.25 The brain abscess of the patient reported here recurred 2 weeks after his initial presentation at SickKids. The focus of the recurrence was the brain abscess, and the negative microbiology was attributed to the long-term use of broad-spectrum antibiotics.
In summary, the lower left second primary molar seems to have produced a brain abscess that resulted in an emergency helicopter flight from Timmins to Toronto, 2 brain surgeries, a dental extraction under general anesthesia, and an extensive and ongoing period of rehabilitation. Most importantly, this 11-year-old boy was saved from an infection with a high mortality and morbidity rate. Dentists are reminded that progressive neurological symptoms can be indicative of a brain abscess, and patients presenting with these symptoms should be promptly referred to an emergency department for care and assessed for any possible sources of dental infection.
Conclusion
Brain abscesses can be caused by dental infections, and treatment of odontogenic infections may avoid the development of a potentially life-threatening condition. Despite the low incidence of brain abscesses, a dental source of infection should be considered in the differential diagnosis of the cause of brain abscesses.
THE AUTHORS
References
- Sheehan JP, Jane JA Jr, Ray DK, Goodkin HP. Brain abscess in children. Neurosurg Focus. 2008;24(6):E6.
- Muzumdar D, Jhawar S, Goel A. Brain abscess: an overview. Int J Surg. 2011;9(2):136-44. Epub 2010 Nov 16.
- Carpenter J, Stapleton S, Holliman R. Retrospective analysis of 49 cases of brain abscess and review of the literature. Eur J Clin Microbiol Infect Dis. 2007;26(1):1-11.
- Tattevin P, Bruneel F, Clair B, Lellouche F, de Broucker T, Chevret S, et al. Bacterial brain abscesses: a retrospective study of 94 patients admitted to an intensive care unit (1980 to 1999). Am J Med. 2003;115(2):143-6.
- Yang SY. Brain abscess: a review of 400 cases. J Neurosurg. 1981;55(5):794-9.
- Alderson D, Strong AJ, Ingham HR, Selkon JB. Fifteen-year review of the mortality of brain abscess.Neurosurgery. 1981:8(1):1-6.
- Goodkin HP, Harper MB, Pomeroy SL. Intracerebral abscess in children: historical trends at Children's Hospital Boston. Pediatrics. 2004;113(6):1765-70.
- Azenha MR, Homsi G, Garcia IR Jr. Multiple brain abscess from dental origin: case report and literature review. Oral Maxillofac Surg. 2011;[Epub ahead print] DOI 10.1007/s10006-011-0308-3.
- Frazier JL, Ahn ES, Jallo GI. Management of brain abscesses in children. Neurosurg Focus. 2008;24(6):E8.
- Zhang J, Stringer MD. Ophthalmic and facial veins are not valveless. Clin Experiment Ophthalmol. 2010;38(5):502-10. Epub 2010 May 10.
- Fischer EG, McLennan JE, Suzuki Y. Cerebral abscess in children. Am J Dis Child. 1981;135(8):746-9.
- Baker SB, Weinzweig J, Bartlett SP, Whitaker LA. Brain abscess as a complication of orthognathic surgery: diagnosis, management and pathophysiology. Plast Reconstr Surg. 1999;104(2):480-2.
- Nishihara J, Takeuchi Y, Miki T, Itoh M, Nagahata S. Anatomical study on valves of human facial veins. J Craniomaxillofac Surg. 1995;23(3):182-6.
- Erickson SJ, Hendrix LE, Massaro BM, Harris GJ, Lewandowski MF, Foley WD, et al. Color Doppler flow imaging of the normal and abnormal orbit. Radiology. 1989;173(2):511-6.
- Gelabert-Gonzalez M, Serramito-Garcia R, Garcia-Allut A, Cutrin-Prieto J. Management of brain abscess in children. J Paediatr Child Health 2008;44(12):731-5. Epub 2008 Nov 18.
- Tseng JH, Tseng MY. Brain abscess in 142 patients: factors influencing outcome and mortality. Surg Neurol. 2006;65(6):557-62.
- Ewald C, Kuhn S, Kalff R. Pyogenic infections of the central nervous system secondary to dental affections – a report of six cases. Neurosurg Rev 2006; 29:163-6, discussion 166-7. Epub 2006 Feb 24.
- Corson MA, Postlethwaite KP, Seymour RA. Are dental infections a cause of brain abscess? Case report and review of the literature. Oral Dis. 2001;7(1):61-5.
- Otto M. For want of a dentist: Pr. George’s boy dies after bacteria from tooth spread to brain. The Washington Post 2007 Feb 28. Availble: http://www.washingtonpost.com/wp-dyn/content/article/2007/02/27/AR2007022702116_pf.html. (accessed 2012 April 23)
- Wolf J, Curtis N. Brain abscess secondary to dental braces. Pediatr Infect Dis J. 2008;27(1):84-5.
- Morita E, Narikiyo M, Nishimura E, Yano A, Tanabe C, Sasaki H, et al. Molecular analysis of age-related changes of Streptococcus anginosus and Streptococcus mitis in saliva. Oral Microbiol Immunol. 2004;19:386-389.
- Al Masalma M, Lonjon M, Richet H, Dufour H, Roche PH, Drancourt M, et al. Metagenomic analysis of brain abscesses identifies specific bacterial associations. Clin Infect Dis. 2012;54(2):202-10. Epub 2011 Dec 5.
- Petti CA, Simmons KE, Bender J, Blaschke A, Webster KA, Conneely MF, et al. Culture-negative intracerebral abscesses in children and adolescents from Streptococcus anginosus group infection: a case series. Clin Infect Dis. 2008;46(10):1578-80.
- Okayama H, Nagata E, Ito H, Oho T, Inoue M. Experimental abscess formation caused by human dental plaque. Microbiol Immunol. 2005;49(5):399-405.
- Madhugiri VS, Sastri BV, Srikantha U, Banerjee AD, Somanna S, Devi BI, Chandramouli BA, et al. Focal intradural brain infections in children: an analysis of management and outcome. Pediatr Neurosurg. 2011;47(2):113-24. Epub 2011 Sep 2.
