Abstract
Background:
The role of oncogenic subtypes of the human papillomavirus (HPV) in the pathogenesis of cervical dysplasia, cervical cancer, anal intraepithelial neoplasia, penile intraepithelial neoplasia, vaginal intraepithelial neoplasia and oropharyngeal carcinomas (OPC) is well documented. The classification and management of HPV-positive OPC (HPV+ OPC) has been modified because of differences in the molecular, biological and clinical behaviour of this disease compared with HPV-negative OPC (HPV− OPC) and conventional oral squamous cell carcinoma (OSCC).
Description
HPV+ OPC is associated with significant morbidity, mortality and cost burden on individual patients and the health care system. Preventive measures and efficient screening programs aim to reduce this burden, and oral health care providers are expected to play a pivotal role in this context. Just as they are involved in tobacco and alcohol counseling and cessation, they should support such public health initiatives as HPV screening, detection of potentially premalignant and malignant lesions, patient education about the risk of HPV infection and promotion of HPV vaccination.
Practical Implications:
The advent of HPV vaccines has modified the epidemiologic landscape and the management of cervical dysplasia and cancer. Similar changes are expected with HPV+ OPC. In this paper, we review HPV+ OPC and discuss how oral health care providers can be effectively involved in the fight against this disease.
Introduction
Human papillomavirus (HPV) is a double-stranded DNA virus infecting human cutaneous and mucosal epithelial cells.1 Among the 200 identified HPV subtypes, more than 40 are known to be transmitted directly via sexual contact or by vertical transmission, such as from mother to child.2,3
Although most HPV types are non-oncogenic (low risk), to date 15 oncogenic types (high risk) have been associated with the development of uterine cervix, anogenital and oropharyngeal malignancies as represented in Table 1.2,4-6 HPV 16 and 18 are responsible for HPV-related oral and oropharyngeal cancer (OPC). Although epidemiologic data show a rise in HPV-related head and neck cancers, we expect to modify this rise by increasing HPV vaccinations. However, despite free school-based vaccinations since 2017 in all Canadian provinces and territories for both genders, rates of vaccine uptakes and completion remain suboptimal.7,8 Therefore, oral health care providers must get involved in patient education to improve and complete the uptake of HPV vaccination.9 The rationale for this review is to reduce the burden of HPV-associated cancers in Canada and North America as a whole and to improve the HPV vaccination uptake as well as to intervene to increase the participation of medical and dental health care professionals in HPV-related OPC prevention. We strive to raise awareness among health care professionals and researchers, encouraging their active participation in the battle against HPV-positive OPC (HPV+ OPC).
|
HPV group |
Risk |
Clinical association |
|---|---|---|
| 6, 11, 42, 43, 44 | Low | External genital warts |
| 2, 4, 40 | Low | Verruca vulgaris (skin wart) |
| 6, 11 | Low | Condyloma acuminatum |
| 13, 32 | Low | Focal epithelial hyperplasia (Heck’s disease) |
| 2, 6, 7, 11, 16, 18, 32, 57 | High | Oral papillomas |
| 77 | Low | Keratoacanthoma |
| 6, 11 | Low | Laryngeal papillomas |
| 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 59, 68 | High | Oropharyngeal carcinoma, all types isolated in cervical cancer, anogenital cancer |
| 75–77 | High | Warts in kidney transplant patients |
Methods
Electronic searching of the Cumulative Index for Nursing and Allied Health Literature (CINAHL), Embase (Ovid), Web of Science, MEDLINE (Ovid) and Scopus was undertaken to identify articles about HPV+ OPC until June 2022. Relevant key terms, such as immunization, vaccination, epidemiology, diagnosis, prevention, early detection, screening, treatment/management, prognosis of human papilloma virus and OPC used in each database were identified and applied.
We included studies investigating HPV+ OPC prevention, diagnosis, treatment and vaccination. No language restriction was applied. All the articles identified in the search were transferred into EndNote 20 for removal of duplicates and reference management. In addition to the automated search strategies, reference lists of retrieved articles and existing reviews were searched for additional studies. Up to 3 attempts were made to contact authors of unpublished data or results.
The studies were screened and reviewed by all the authors to extract all the relevant and accurate information for presentation in this paper.
Epidemiology of HPV Infection
The worldwide prevalence of HPV infection in the general population ranges from 2% to 44%.10 This wide disparity can be explained by variations in the population studied, participant demographics, the sensitivity of the HPV assay used and the sites from which samples are collected. The prevalence is higher among young adults, and HPV affects over 50% of sexually active women at some point.11-13 In the United States and Canada, an estimated 27% of young women and a third of all women are infected with at least 1 strain of HPV.14,15 Although the prevalence of HPV infection tends to decline with increasing age, a second peak has been reported among women >50 years.16-18 HPV infection in men is less studied and appears to be less frequent, with no bimodal age-specific distribution, as in women.18,19 A study of heterosexuality active males20 found that the 24-month cumulative incidence of genital HPV infection in male university students aged 18–20 was 62.4%.
Benign HPV-Related Head and Neck Lesions
Most common benign HPV-related lesions of the oral cavity are oral squamous papilloma (caused by HPV types 6 and 11),21,22 verruca vulgaris (types 2 and 4), multifocal epithelial hyperplasia (Heck disease; types 13 and 32) and condyloma acuminatum (types 6 and 11).2,23 Clinically, oral lesions have no potential for malignant transformation and present as well-circumscribed, solitary, coalescing or multifocal papillary lesions that can be white, pink or red depending on their location and degree of keratinization.24 In some cases, oral lesions regress with time; others are treated with conservative surgical excision.2,23
Malignant HPV-Related Head and Neck Lesions
The oropharynx is the most common site of HPV-related malignancy in the head and neck region. The anatomic border of the oropharynx includes the base of the tongue, tonsillar region, soft palate and posterior pharyngeal wall (Figure 1). However, HPV-related malignancy can also occur in the oral cavity, sinonasal cavity, larynx, hypopharynx and nasopharynx.25
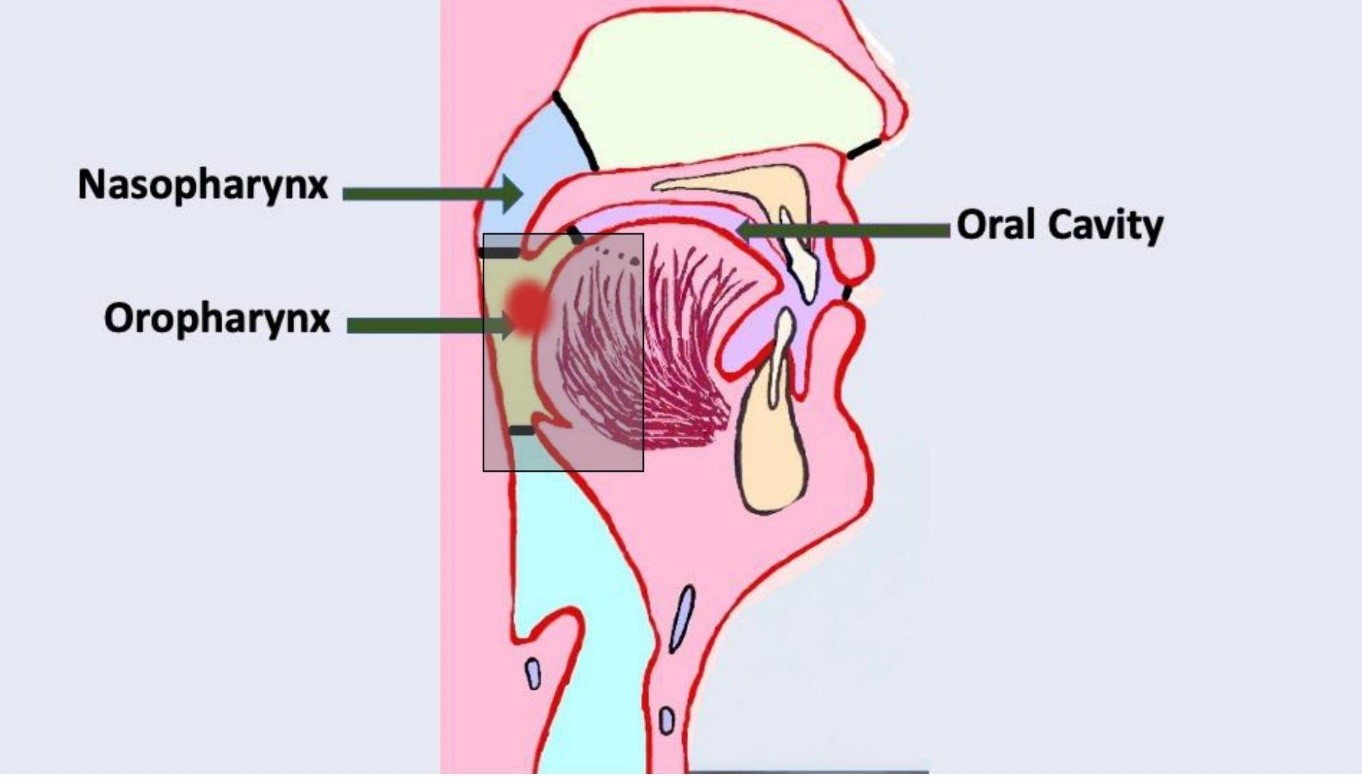
Figure 1: Location of the oral cavity and oropharynx. Shaded box highlights site for human papillomavirus-related oropharyngeal carcinoma. Diagram created by Dr. Gordon.
Epidemiology of HPV+ OPC, HPV− OPC and oral squamous cell carcinoma
Oral squamous cell carcinoma (OSCC) is the most common non-dermatologic malignancy of the head and neck. Like HPV− OPC, OSCC is most common in men, aged 60–70 years, those with a long history of smoking and/or alcohol use and those with lower socioeconomic status.26 The prevalence of HPV in OSCC ranges from 3.9% to 34.5% while the overall weighted prevalence of HPV in head and neck cancers is 20.2% worldwide and 17% in the United States.27 Variation in prevalence may be attributed to multiple factors, including site of the tumour (e.g., oral cavity versus oropharynx), differences in studied populations, methods of tissue sampling and testing technology.27 A drastic shift in the epidemiology of head and neck cancers has been observed with the decline in the incidence of smoking-related cancers and a rise in HPV-related cancers.28 A recent study revealed that infection with HPV 16, but not HPV 18, may increase the risk of developing cancers of the floor of the mouth, gingiva, tongue or palate.29 Therefore, health care providers should consider emerging risk factors, such as HPV infection, along with traditional factors, such as tobacco and alcohol use, in OSCC management.28 In Canada, the incidence of OSCC decreased from 6.2 per 100 000 population in 1997 to 4.2 in 2012. This can be attributed mainly to the reduction of smoking among those aged ≥15 years from 25.5% in 1999 to 13% in 2015.20 HPV+ OPC is more likely to affect Caucasian males in their 40s and 50s, those of higher socioeconomic status,30,31 those who were younger at first sexual contact, men who have sex with men who have higher number of lifetime oral or anal sex partners (>5), those with a higher number of lifetime vaginal sex partners (>25) and those with concurrent genital HPV infection.32,33 The risk of oral and oropharyngeal HPV infection increases with cigarette smoking and/or marijuana and alcohol use. Theoretically, the pro-inflammatory effects of smoking increase the persistence of HPV at these sites.34 Sexual partners of patients with HPV+ OPC may have a higher prevalence of oral HPV infection. It is unclear whether such an infection may lead to clinical disease or may resolve spontaneously.33 In any case, it is strongly recommended that these individuals be followed closely to better understand and estimate their risk of developing HPV+ OPC.35
The rise in the incidence of HPV+ OPC in the United States has made it the most common HPV-related malignancy,33,36 and, according to the Centers for Disease Control and Prevention, it has exceeded the incidence of HPV-associated uterine cervical cancers.37 If these trends become global, the rate of HPV+ OPC in males will surpass the rate of cervical cancer in females.20 Although recent studies revealed lower HPV+ OPC incidence among females, that rate is increasing in certain subgroups, such as non-Hispanic white women.38 Because of the limited data on HPV prevalence in OSCC and its role in prognosis, OSCC is still staged and treated regardless of HPV status.39 However, it is now recognized that the prognosis for HPV+ OPC is better than OSCC and HPV− OPC. This change in paradigm is reflected in the 8th edition of the American Joint Committee on Cancer staging manual and the 2017 World Health Organization (WHO) Classification of Head and Neck Tumours, which consider OSCC, HPV+ OPC and HPV− OPC as 3 distinct diagnostic entities. This monumental shift in the stratification protocol of head and neck cancers has improved estimates of prognosis and led to more precise assessment of treatment outcomes.40-42
Molecular Role of HPV in Cancer Development
HPV+ OPC and anogenital cancer have similar carcinogenic mechanisms but differ in terms of epidemiologic factors, HPV type, mutational profile, cell of origin, treatment response and clinical behaviour, suggesting that they are distinct from each other at the molecular level.43 In an analysis of an expanded cohort from the Cancer Genome Atlas program, TRAF3 or CYLD mutations or deletions were found in almost 30% of HPV+ OPC tumours, while these mutations are extremely rare in cervical cancer.44,45 Clinically, HPV+ OPC responds better to treatment than HPV-related cervical cancer, suggesting that the underlying molecular variations play a significant role in the unique properties of their respective epithelial sites of infection, different clinical signs and symptoms, patterns of metastasis and treatment response.43 Therefore, caution is necessary when comparing these HPV-associated diseases (Table 2).
|
Comparators |
HPV+ OPC |
HPV− OPC |
Cervical cancer |
|---|---|---|---|
| Incidence | Increasing | Decreasing | Decreasing |
| Prevalence | Increased with higher socioeconomic status | Decreased in western countries | Increased with lower socioeconomic status |
| Sex | >70% male | >85.8% male | 100% female |
| Etiology | Sexually transmitted HPV 16 and HPV 18 | Tobacco and alcohol | Virtually all caused by HPV |
| HPV genotype | > 95% HPV 16; rarely HPV 18 | Not associated | 50% HPV 16, 20% HPV 18 |
| Premalignant lesions | Unknown | Leukoplakia, erythroplakia | Cervical intraepithelial neoplasia 1–3 |
| Screening tests available | No | No | Yes |
| 5-year survival rate | >75% | 11.1% | <70% |
| TRAF3/CYLD mutations | Approximately 30% (low) | High | Rare |
| Treatment sensitivity to chemotherapy and radiation | High | Low | Moderate |
Our understanding of HPV-induced transformation has relied heavily on research of cervical premalignant lesions that suggest a canonical model of HPV carcinogenesis wherein establishment and maintenance of HPV infection are thought to parallel the HPV normal life cycle, but persistent basal or stem-cell infection initiates carcinogenesis. As cervical cells progress from early dysplasia to in-situ disease (cervical intraepithelial neoplasia [CIN] 1 to 3), the HPV genome integrates and disrupts the HPV E2 gene, which normally act as a negative regulator of the viral oncoproteins E6 and E7; this disruption leads to uncontrolled expression of HPV oncoproteins (E6 and E7).46-49 The expression of these oncoproteins occurs in superficial layers of epithelium in HPV infection. However, during carcinogenesis, premalignant cells with high E6 and E7 expression persist at the basal layer of the epithelium where immune-mediated clearance has not occurred.
Increased expression of HPV oncoproteins inactivates the major human tumour-suppressor genes, TP53 and RB, leading to genomic instability, resistance to apoptosis and dysregulated cell-cycle control. Whether this model applies to HPV+ OPC, in which the HPV has been integrated into the genome, has been an issue of controversy. However, it is evident that this model does not apply to HPV+ OPC in which HPV has not been integrated into the genome.50 Other molecules have been suggested to play a role in the development of HPV+ OPC. These include APOBEC (apolipoprotein B mRNA editing enzyme, catalytic polypeptide-like), activating mutations of PI3KCA,51 as well as disruption of TRAF3 and CYLD as inhibitors of NF-kB and activators of type I interferon.52-55 Detection of HPV carcinogenesis in the tonsil is difficult because of the lack of defined and detectable premalignant lesions. Moreover, the lack of tight epithelial junctions and incomplete basement membrane in tonsillar crypts makes it challenging to distinguish invasive cancer from intra-epithelial dysplastic changes.56,57 In fact, the College of American Pathologists’ 2017 guidelines state that in-situ disease in HPV+ OPC is “non-existent.”58
Clinical Presentation and Diagnosis of HPV+ OPC
HPV+ OPC can be challenging to detect on physical examination or with CT scan, MRI or laryngoscopy.59 Therefore, diagnosis is often established at late stages. The primary malignancy can cause asymmetric enlargement of the oropharyngeal lymphatic tissues. A common clinical presentation of HPV+ OPC is enlarged cervical lymph nodes, representing metastasis of the primary lesion.33 Other symptoms that may be associated with primary HPV+ OPC include otalgia, dysphagia, odynophagia and persistent sore throat; however, these symptoms are more commonly associated with primary lesions of HPV− OPC and may overlap with other malignancies involving the oropharynx.60 The clinical presentation of a patient with HPV+ OPC is demonstrated in Figure 2.
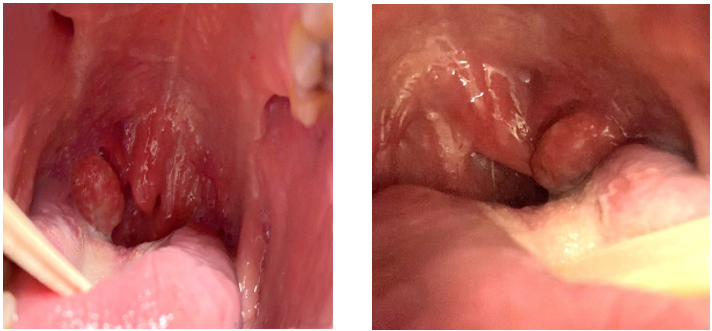
Figure 2: Clinical presentation of human papillomavirus positive oropharyngeal carcinomas.
Diagnosis relies mainly on history, clinical examination, endoscopic assessment, medical imaging and biopsy. Imaging may include CT, MRI, positron emission tomography and/or ultrasound. Ultrasound-guided fine needle aspiration is the most common biopsy procedure used to confirm the presence of metastatic disease in an enlarged cervical lymph node. Early lesions are best diagnosed with endoscopic systems with narrow band imaging.61
Treatment approaches include single and multimodal therapy depending on the location of the primary lesion, lesion extension and TNM staging. Resection of the primary tumour is performed using traditional surgical methods or transoral robotic surgery. It is important to note that surgical treatment of the primary lesion is not necessary in some cases. Suspected or confirmed metastases in cervical lymph nodes may be treated by surgical neck dissection and/or radiation therapy. Radiation therapy with or without adjunctive chemotherapy is planned based on the site involved and pathologic stage of the tumour.
A comparison of management approaches of HPV+ OPC, HPV− OPC and other oral cavity carcinomas is represented in Table 3.
|
Comparators |
HPV+ OPC |
HPV− OPC |
Oral cavity carcinomas |
|---|---|---|---|
| Prognosis | Favourable | Worse | Depends on stage and site |
| Surgical excision | Removal of cancerous tissue | Partial or total removal depending on extent | Excision of tumour with healthy tissue margin |
| Radiation/ chemotherapy or both | Intensity-modulated radiation therapy combined with chemotherapy with cisplatin | Primary treatment or in combination with surgery | Primary surgical treatment or in combination of radiation therapy |
| Immunotherapy | Immune checkpoint inhibitors, such as pembrolizumab and nivolumab | Ongoing research to explore the effectiveness | Role may vary depending on the specific carcinoma type and patient characteristics |
| Response to treatment | Often good | Less responsive | Variable |
| Prevention strategies | HPV vaccination | Tobacco cessation, alcohol moderation | Lifestyle changes, early detection |
| Recurrence monitoring | Required | Required | Required |
In addition to a better prognosis for HPV+ OPC compared with HPV− OPC, the overall 5-year survival rate is estimated at 62% and 33%, respectively.62 Like most other human cancers, the clinical stage of the disease at the time of diagnosis plays a major role in determining the prognosis.
Prevention of HPV+ OPC: the Role of HPV Vaccination and the Oral Health Care Team
Oral health care providers are positioned to play a key role in the prevention and early detection of HPV-related head and neck cancers.63 Their involvement in an interprofessional approach will be of critical importance in reducing the burden of disease.33 Clinical histories and medical health questionnaires should be updated by dental teams to include known risk factors for HPV+ OPC development. Along with questions pertaining to risk factors, such as tobacco and alcohol use, oral health care providers should obtain a sexual history and vaccination status to elucidate the patient’s overall risk of developing oral and/or OPC.64
Clinical examination should include palpation of the cervical lymph nodes and thorough examination (visual and tactile if possible) of all soft tissues in the oral cavity and accessible oropharyngeal sites, including the base of the tongue, pharyngeal tonsils, tonsillar pillars, soft palate, uvula and pharyngeal walls to facilitate early detection of oral and oropharyngeal lesions.
The benefit of an early diagnosis — through HPV screening of all patients using biopsies taking an adequate sample — outweighs the risk of non-diagnostic or false positive cases.65 As we know, false positive results can lead to invasive and costly testing that may be unnecessary; therefore, dentists should consider the precision of biopsy techniques. Unlike cervical cancer, no Food and Drug Administration-approved screening tests are available to determine HPV+ OPC risk using HPV DNA or mRNA. Research efforts currently focus on developing screening tools for detection of HPV+ OPC in otherwise healthy patients using salivary rinses and/or oral swabs to obtain potential samples.66,67 Furthermore, a novel clinically validated circulating tumour-tissue-modified HPV DNA blood test, named NavDx, has been developed to aid in the detection of HPV-driven cancer. This highly accurate blood test reliably uncovers the presence of HPV+ head and neck carcinoma during care.6
Several HPV vaccines have been developed and are currently in use.68-70 The 9-valent vaccine, Gardasil 9 (Merck Sharp & Dohme LLC, Rahway, N.J., USA), is available for prevention of infection with HPV types 6, 11, 16, 18, 31, 33, 45, 52 and 58.71 Indeed, there has been a worldwide decrease in HPV infections since the implementation of prophylactic HPV vaccination.72,73
The American Dental Association has urged oral health care providers to participate in HPV prevention by educating their patients and promoting HPV vaccination.74 Studies assessing professionals’ willingness and confidence in discussing HPV prevention and vaccination have highlighted the need to provide more frequent continuing education in this evolving area. Although dental professionals are undoubtedly interested in their patients’ health and recognize the need to promote HPV vaccination, there are barriers, such as difficulty discussing sexual behaviour, lack of adequate knowledge about the role of HPV and preventive strategies, vaccine administration and safety and liability concerns.63,64,69
Training oral health care providers to perform HPV screening, prevention and patient education is essential to reduce knowledge gaps. This must begin in the undergraduate dental curriculum75 and be maintained through continuing education.
Until recently, few oral health care providers have been trained or licensed to administer vaccines. However, since 2019, dentists in Oregon, USA, have been authorized to prescribe and administer any vaccine.64,72,76 Recently, because of the COVID-19 pandemic, dentists in some Canadian provinces, many American states and other parts of the world became authorized to provide inoculations against SARS-CoV-2.77-79 In the United States, vaccination training has become a part of the undergraduate dental curriculum at several dental schools and is offered in several continuing dental education courses.80,81 In Canada, as of 2021, there have been policy discussions with provincial regulatory bodies regarding administering HPV vaccinations and providing requisite didactic and hands-on training to safely perform such a procedure.82
Health Canada Guidelines on HPV Vaccines
Currently, it is estimated that 75% of Canadians are prone to HPV infection at some time based on data from the Public Health Agency of Canada.83 Considering this high risk, even 1 dose of HPV vaccine (9vHPV) at any age is expected to provide some benefit compared with no vaccination. Based on current Health Canada guidelines, 9vHPV is strongly recommended for all individuals 9–26 years of age; those ≥27 years who are at risk of exposure to HPV may receive the vaccine after consulting with a health care provider. For immunocompromised patients, a 3-dose schedule of 9vHPV vaccine is recommended. Those who have previously received a different HPV vaccine, such as 2vHPV, should consider a dose of 9vHPV vaccine for added protection against other HPV types, after discussion with their health care provider. For a 2-dose or 3-dose HPV vaccine schedule, the first and last doses should be separated by at least 6 months. HPV vaccines have been proven safe and well tolerated, with an adverse event occurring in 1% to <10% of vaccine recipients. In Canada, vaccine providers are required by law to report adverse events following immunization. Currently, all Canadian provinces and territories have publicly funded, school-based HPV vaccination programs for children in grades 4–8; those who miss this immunization can get vaccinated in catch-up programs under health plans, although eligibility for these programs varies based on age and gender.83
Although this current study provides a comprehensive understanding of HPV+ OPC and current treatment and prevention strategies, it has certain strengths and limitations. The major strength of this study is its multidisciplinary perspective on knowledge of HPV+ OPC including literature along with the opinions of practitioners involved in patient care. However, the potential bias in study selection and synthesis of the findings from diverse study designs cannot be ignored.
Conclusion
Given the significant morbidity, mortality and cost burden associated with HPV+ OPC, prevention and screening of HPV infections and HPV-related cancers remain of utmost importance. Oral health care providers can play an essential role in the fight against HPV-related disease by implementing adequate screening and early detection of HPV-related lesions, educating patients regarding risk factors for HPV-related cancers and promoting HPV vaccination. With the development of clear guidelines and adequate training, dentists can be among the health care providers capable of administering HPV vaccines.
THE AUTHORS
Corresponding author: Dr. Firoozeh Samim, Faculty of dental medicine and oral health science, McGill University, 2001 McGill College Ave, Montreal, QC H3A 1G1. Email: firoozeh.samim@mcgill.ca
The authors have no declared financial interests.
This article has been peer reviewed.
References
- Trottier H, Franco EL. The epidemiology of genital human papillomavirus infection. Vaccine. 2006;24 Suppl 1:S1-15.
- Gordon SC, Ganatra S, Sroussi HY. Viral infections of the mouth. Medscape Reference. (updated 2018) https://emedicine.medscape.com/article/1079920-overview?form=fpf (accessed 2024 Dec 22).
- Dassi L, Annunziata C, Botti C, Micillo A, Cerasuolo A, Starita N, et al. Detection of human papillomaviruses in the nasopharynx of breastfed infants: new findings and meta-analysis. Viruses. 2020;12(10):1119.
- Cubie HA. Diseases associated with human papillomavirus infection. Virology. 2013;445(1-2):21-34.
- Dias TR, Santos JMO, da Costa RMG, Medeiros R. Long non-coding RNAs regulate the hallmarks of cancer in HPV-induced malignancies. Crit Rev Oncol Hematol. 2021:103310.
- Chera BS, Kumar S, Shen C, Amdur R, Dagan R, Green R, et al. Plasma circulating tumor HPV DNA for the surveillance of cancer recurrence in HPV-associated oropharyngeal cancer. J Clin Oncol. 2020;38(10):1050-8.
- Duval B, Gilca V, McNeil S, Dobson S, Money D, Gemmill IM, et al. Vaccination against human papillomavirus: a baseline survey of Canadian clinicians’ knowledge, attitudes and beliefs. Vaccine. 2007;25(45):7841-7.
- Goyette A, Yen GP, Racovitan V, Bhangu P, Kothari S, Franco EL. Evolution of public health human papillomavirus immunization programs in Canada. Curr Oncol. 2021;28(1):991-1007.
- Dubé E, Gagnon D, Clément P, Bettinger JA, Comeau JL, Deeks S, et al. Challenges and opportunities of school-based HPV vaccination in Canada. Hum Vaccin Immunother. 2019;15(7-8):1650-5.
- Giuliano AR, Tortolero-Luna G, Ferrer E, Burchell AN, de Sanjose S, Kjaer SK, et al. Epidemiology of human papillomavirus infection in men, cancers other than cervical and benign conditions. Vaccine. 2008;26(Suppl 10):K17-28.
- Sethi S, Ali A, Ju X, Antonsson A, Logan R, Canfell K, et al. A systematic review and meta-analysis of the prevalence of human papillomavirus infection in Indigenous populations - a global picture. J Oral Pathol Med. 2021;50(9):843-54.
- Baseman JG, Koutsky LA. The epidemiology of human papillomavirus infections. J Clin Virol. 2005;32 Suppl 1:S16-24.
- Roteli-Martins CM, de Carvalho NS, Naud P, Teixeira J, Borba P, Derchain S, et al. Prevalence of human papillomavirus infection and associated risk factors in young women in Brazil, Canada, and the United States: a multicenter cross-sectional study. Int J Gynecol Pathol. 2011;30(2):173-84.
- Reiter PL, McRee AL. HPV infection among a population-based sample of sexual minority women from USA. Sex Transm Infect. 2017;93(1):25-31.
- Strickler HD, Burk RD, Fazzari M, Anastos K, Minkoff H, Massad LS, et al. Natural history and possible reactivation of human papillomavirus in human immunodeficiency virus-positive women. J Natl Cancer Inst. 2005;97(8):577-86.
- Herrero R, Hildesheim A, Bratti C, Sherman ME, Hutchinson M, Morales J, et al. Population-based study of human papillomavirus infection and cervical neoplasia in rural Costa Rica. J Natl Cancer Inst. 2000;92(6):464-74.
- Hao S, Wang C, Liu S, He J, Jiang Y. HPV genotypic spectrum in Jilin province, China, where non-vaccine-covered HPV53 and 51 are prevalent, exhibits a bimodal age-specific pattern. PLoS One. 2020;15(3):e0230640.
- Bettampadi D, Villa LL, Ponce EL, Salmeron J, Sirak BA, Abrahamsen M, et al. Oral human papillomavirus prevalence and type distribution by country (Brazil, Mexico and the United States) and age among HPV infection in men study participants. Int J Cancer. 2020;146(11):3026-33.
- Van Doornum GJ, Prins M, Juffermans LH, Hooykaas C, van den Hoek JA, Coutinho RA, et al. Regional distribution and incidence of human papillomavirus infections among heterosexual men and women with multiple sexual partners: a prospective study. Genioturin Med. 1994;70(4):240-6.
- Partridge JM, Hughes JP, Feng Q, Winer RL, Weaver BA, Xi LF et al. Genital human papillomavirus infection in men: incidence and risk factors in a cohort of university students. J Infect Dis. 2007 Oct 15;196(8):1128-36.
- Beutner KR, Tyring S. Human papillomavirus and human disease. Am J Med. 1997;102(5A):9-15.
- Syrjänen S. Human papillomavirus infections and oral tumors. Med Microbiol Immunol. 2003;192(3):123-8.
- Gordon SC, Patel MC, Cabay RJ, Sroussi HY. Benign diseases associated with human papillomavirus infection. In: Radosevich J, editor. HPV and cancer. Dordrecht: Springer; 2012. p. 131-62.
- Syrjänen S. Oral manifestations of human papillomavirus infections. Eur J Oral Sci. 2018;126 Suppl 1:49-66.
- Liu X, Gao XL, Liang XH, Tang YL. The etiologic spectrum of head and neck squamous cell carcinoma in young patients. Oncotarget. 2016;7(40):66226-38.
- Warnakulasuriya S. Living with oral cancer: epidemiology with particular reference to prevalence and life-style changes that influence survival. Oral Oncol. 2010;46(6):407-10.
- Isayeva T, Li Y, Maswahu D, Brandwein-Gensler M. Human papillomavirus in non-oropharyngeal head and neck cancers: a systematic literature review. Head Neck Pathol. 2012;6(Suppl 1):104-20.
- McDermott JD, Bowles DW. Epidemiology of head and neck squamous cell carcinomas: impact on staging and prevention strategies. Curr Treat Options Oncol. 2019;20(5):1-13.
- Giraldi L, Collatuzzo G, Hashim D, Franceschi S, Herrero R, Chen C, et al. Infection with human papilloma virus (HPV) and risk of subsites within the oral cancer. Cancer Epidemiol. 2021;75:102020.
- Gillison ML, D'Souza G, Westra W, Sugar E, Xiao W, Begum S, et al. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J Natl Cancer Inst. 2008;100(6):407-20.
- Ghazawi FM, Lu J, Savin E, Zubarev A, Chauvin P, Sasseville D, et al. Epidemiology and patient distribution of oral cavity and oropharyngeal SCC in Canada. J Cutan Med Surg. 2020;24(4):340-9.
- Kreimer AR, Chaturvedi AK, Alemany L, Anantharaman D, Bray F, Carrington M, et al. Summary from an international cancer seminar focused on human papillomavirus (HPV)-positive oropharynx cancer, convened by scientists at IARC and NCI. Oral Oncol. 2020;108:104736.
- Timbang MR, Sim MW, Bewley AF, Farwell DG, Mantravadi A, Moore MG. HPV-related oropharyngeal cancer: a review on burden of the disease and opportunities for prevention and early detection. Hum Vaccin Immunother. 2019;15(7-8):1920-8.
- Sonawane K, Suk R, Chiao EY, Chhatwal J, Qiu P, Wilkin T, et al. Oral human papillomavirus infection: differences in prevalence between sexes and concordance with genital human papillomavirus infection, NHANES 2011 to 2014. Ann Intern Med. 2017;167(10):714-24.
- Tsao AS, Papadimitrakopoulou V, Lin H, Guo M, Lee JJ, Holsinger FC, et al. Concordance of oral HPV prevalence between patients with oropharyngeal cancer and their partners. Infect Agent Cancer. 2016;11:21.
- Ramqvist T, Dalianis T. Oropharyngeal cancer epidemic and human papillomavirus. Emerg Infect Dis. 2010;16(11):1671-7.
- Reasons to get HPV vaccine. Atlanta: Centers for Disease Control and Prevention; 2021. Available: https://www.cdc.gov/hpv/vaccines/reasons-to-get.html (accessed Jan 22 2025).
- Zavras AI, Shanmugam P, Shetty D, Dolecek TA, Kaste LM. Oral and pharyngeal cancer in women. Dent Clin North Am. 2013;57(2):339-55.
- Fakhry C, D’Souza G. Discussing the diagnosis of HPV-OSCC: Common questions and answers. Oral Oncol. 2013;49(9):863-71.
- Laskar SG, Swain M. HPV positive oropharyngeal cancer and treatment deintensification: how pertinent is it? J Cancer Res Ther. 2015;11(1):6-9.
- Lydiatt WM, Patel SG, O’Sullivan B, Brandwein MS, Ridge JA, Migliacci JC, et al. Head and neck cancers — major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67(2):122-37.
- Rooper LM, Windon MJ, Hernandez T, Miles B, Ha PK, Ryan WR, et al. HPV-positive squamous cell carcinoma of the larynx, oral cavity, and hypopharynx: clinicopathologic characterization with recognition of a novel warty variant. Am J Surg Pathol. 2020;44(5):691-702.
- Berman TA, Schiller JT. Human papillomavirus in cervical cancer and oropharyngeal cancer: one cause, two diseases. Cancer. 2017;123(12):2219-29.
- Hajek M, Sewell A, Kaech S, Burtness B, Yarbrough WG, Issaeva N. TRAF3/CYLD mutations identify a distinct subset of human papillomavirus-associated head and neck squamous cell carcinoma. Cancer. 2017;123(10):1778-90.
- Leemans CR, Snijders PJ, Brakenhoff RH. The molecular landscape of head and neck cancer. Nat Rev Cancer. 2018;18(5):269-82.
- Cullen AP, Reid R, Campion M, Lörincz A. Analysis of the physical state of different human papillomavirus DNAs in intraepithelial and invasive cervical neoplasm. J Virol. 1991;65(2):606-12.
- Hudelist G, Manavi M, Pischinger KID, Watkins-Riedel T, Singer CF, Kubista E, et al. Physical state and expression of HPV DNA in benign and dysplastic cervical tissue: different levels of viral integration are correlated with lesion grade. Gynecol Oncol. 2004;92(3):873-80.
- Pinidis P, Tsikouras P, Iatrakis G, Zervoudis S, Koukouli Z, Bothou A, et al. Human papilloma virus’ life cycle and carcinogenesis. Maedica (Bucur). 2016;11(1):48-54.
- Spurgeon ME, Lambert PF. Human papillomavirus and the stroma: bidirectional crosstalk during the virus life cycle and carcinogenesis. Viruses. 2017;9(8):219.
- Hatano T, Sano D, Takahashi H, Oridate N. Pathogenic role of immune evasion and integration of human papillomavirus in oropharyngeal cancer. Microorganisms. 2021;9(5):891.
- Cancer Genome Atlas Research Network, et al. Integrated genomic and molecular characterization of cervical cancer. Nature. 2017;543(7645):378-84.
- Zhang J, Chen T, Yang X, Cheng H, Späth SS, Clavijo PE, et al. Attenuated TRAF3 fosters activation of alternative NF-κB and reduced expression of antiviral interferon, TP53, and RB to promote HPV-positive head and neck cancers. Cancer Res. 2018;78(17):4613-26.
- Lohavanichbutr P, Houck J, Fan W, Yueh B, Mendez E, Futran N, et al. Genomewide gene expression profiles of HPV-positive and HPV-negative oropharyngeal cancer: potential implications for treatment choices. Arch Otolaryngol Head Neck Surg. 2009;135(2):180-8.
- Kondo S, Wakae K, Wakisaka N, Nakanishi Y, Ishikawa K, Komori T, et al. APOBEC3A associates with human papillomavirus genome integration in oropharyngeal cancers. Oncogene. 2017;36(12):1687-97.
- Kano M, Kondo S, Wakisaka N, Wakae K, Aga M, Moriyama‐Kita M, et al. Expression of estrogen receptor alpha is associated with pathogenesis and prognosis of human papillomavirus‐positive oropharyngeal cancer. Int J Cancer. 2019;145(6):1547-57.
- Gelwan E, Malm IJ, Khararjian A, Fakhry C, Bishop JA, Westra WH. Nonuniform distribution of high-risk human papillomavirus in squamous cell carcinomas of the oropharynx. Am J Surg Pathol. 2017;41(12):1722-8.
- Perry ME. The specialised structure of crypt epithelium in the human palatine tonsil and its functional significance. J Anat. 1994;185(Pt 1):111-27.
- Marur S, D'Souza G, Westra WH, Forastiere AA. HPV-associated head and neck cancer: a virus-related cancer epidemic. Lancet Oncol. 2010;11(8):781-9.
- Ni X, Cheng R, Lai S, Zhang L, He S, Zhang Y, et al. Value of narrow band imaging endoscopy in the detection of unknown primary site with cervical lymph node metastasis of squamous cell carcinoma [article in Chinese]. Zhonghua Zhong Liu Za Zhi. 2013;35(9):698-702.
- Carvalho RS, Scapulatempo-Neto C, Curado MP, de Castro Capuzzo R, Teixeira FM, Pires RC, et al. HPV-induced oropharyngeal squamous cell carcinomas in Brazil: prevalence, trend, clinical and epidemiological characterization. Cancer Epidemiol Biomarkers Prev. 2021;30(9):1697-707.
- Ni XG, Wang GQ. The role of narrow band imaging in head and neck cancers. Curr Oncol Rep. 2016;18(2):10.
- Kędzierawski P, Huruk-Kuchinka A, Radowicz-Chil A, Mężyk R, Rugała Z, Sadowski J. Human papillomavirus infection predicts a better survival rate in patients with oropharyngeal cancer. Arch Med Sci. 2020;17(5):1308-16.
- Guadiana D, Kavanagh NM, Squarize CH. Oral health care professionals recommending and administering the HPV vaccine: understanding the strengths and assessing the barriers. PLoS One. 2021;16(3):e0248047.
- Patel S, Koskan A, Spolarich A, Perry M, Flood T. Dental professionals' knowledge, attitudes, and practice behaviors related to human papillomavirus vaccination. J Public Health Dent. 2020;80(1):61-9.
- D'Souza G, McNeel TS, Fakhry C. Understanding personal risk of oropharyngeal cancer: risk-groups for oncogenic oral HPV infection and oropharyngeal cancer. Ann Oncol. 2017;28(12):3065-9.
- Gipson BJ, Robbins HA, Fakhry C, D'Souza G. Sensitivity and specificity of oral HPV detection for HPV-positive head and neck cancer. Oral Oncol. 2018;77:52-6.
- Donà MG, Pichi B, Rollo F, Benevolo M, Latini A, Laquintana V, et al. Human papillomavirus detection in matched oral rinses, oropharyngeal and oral brushings of cancer-free high-risk individuals. Oral Oncol. 2019;91:1-6.
- Harder T, Wichmann O, Klug SJ, van der Sande MAB, Wiese-Posselt M. Efficacy, effectiveness and safety of vaccination against human papillomavirus in males: a systematic review. BMC Med. 2018;16(1):110.
- Aldossri M, Okoronkwo C, Dodd V, Manson H, Singhal S. Determinants of dentists' readiness to assess HPV risk and recommend immunization: a transtheoretical model of change-based cross-sectional study of Ontario dentists. PLoS One. 2021;16(2):e0247043.
- Bruni L, Diaz M, Barrionuevo-Rosas L, Herrero R, Bray F, Bosch FX, et al. Global estimates of human papillomavirus vaccination coverage by region and income level: a pooled analysis. Lancet Glob Health. 2016;4(7):e453-63.
- Petrosky E, Bocchini Jr JA, Hariri S, Chesson H, Curtis CR, Saraiya M, et al. Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccination recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep. 2015;64(11):300-4.
- Villa A, Chmieliauskaite M, Patton LL. Including vaccinations in the scope of dental practice: the time has come. J Am Dent Assoc. 2021;152(3):184-6.
- Kim MA, Han GH, Kim JH, Seo K. Current status of human papillomavirus infection and introduction of vaccination to the national immunization program in Korea: an overview. J Korean Med Sci. 2018;33(52):e331.
- ADA webinar on vaccines and dentists’ role on tap Jan. 27. Chicago: American Dental Association; 2021.
- Poelman MR, Brand HS, Forouzanfar T, Daley EM, Jager DHJ. Prevention of HPV- related oral cancer by dentists: assessing the opinion of Dutch dental students. J Cancer Educ. 2018;33(6):1347-54.
- House bill 2220. Salem: 81st Oregon Legislative Assembly; 2021. Available: https://olis.oregonlegislature.gov/liz/2021R1/Downloads/MeasureDocument/HB2220/Introduced (accessed 2021 Nov. 20).
- Seventh amendment to declaration under the public readiness and emergency preparedness act for medical countermeasures against COVID- 19. Washington, DC: Health and Human Services Department; 2021. Available: https://www.federalregister.gov/documents/2021/03/16/2021-05401/seventh-amendment-to-declaration-under-the-public-readiness-and-emergency-preparedness-act-for (accessed 2021 Apr. 26).
- New FDI World Dental Federation global survey reveals that two-thirds of countries are not allowing dentists to administer COVID-19 vaccines. New York: PR Newswire; 2021. https://www.prnewswire.com/in/news-releases/new-fdi-world-dental-federation-global-survey-reveals-that-two-thirds-of-countries-are-not-allowing-dentists-to-administer-covid-19-vaccines-872245906.html (accessed 2024 Dec. 23).
- Dentists among health professionals included in public health order as B.C. prepares for widespread mass vaccinations. Vancouver: British Columbia College of Oral Health Professionals; 2021.
- Bush E. Dentists to be part of the coronavirus vaccination effort in Washington state. Seattle Times 2021; Jan. 20. Available: https://www.seattletimes.com/seattle-news/health/dentists-to-be-part-of-the-coronavirus-vaccination-effort-in-washington-state/ (accessed 2024 Dec. 23).
- COVID-19 vaccine administration by dental hygienists. Chicago: American Dental Hygienists’ Association; 2021.
- Office of the Chief Dental Officer of Canada. Human papillomavirus and oral health. Can Commun Dis Rep. 2020;46(11/12):380-3. Available: https://www.canada.ca/en/public-health/services/reports-publications/canada-communicable-disease-report-ccdr/monthly-issue/2020-46/issue-11-12-november-5-2020/human-papillomavirus-oral-health.html (accessed 2024 Dec. 23).
- Human papillomavirus (HPV) vaccines: Canadian immunization guide. Ottawa: Government of Canada; 2024. Available: https://www.canada.ca/en/public-health/services/publications/healthy-living/canadian-immunization-guide-part-4-active-vaccines/page-9-human-papillomavirus-vaccine.html (accessed 2024 Dec. 23).







