Abstract
Introduction:
Cannabis continues to garner attention due to increasing legalization globally and its potential effects on public health. Consequently, dental practitioners should be familiar with its impacts on oral health and how to properly manage these impacts in the clinical setting. This review explores the connection between cannabis use and oral health, providing insights into national usage trends, cannabis pharmacology, potential oral health impacts and important considerations for patient management.
Methods
We compiled data on self-reported cannabis use from the Canadian Cannabis Survey (2017–2024) and analyzed changes in cannabis use over time with a 2-tailed z-test. We performed a scoping literature review using multiple databases to examine cannabis pharmacology, dentally relevant drug interactions, oral health effects and dental management strategies.
Results:
Following legalization in 2018, cannabis use in Canada increased by 3.8 percentage points (p < 0.001). Although cannabis may have some dentally relevant drug interactions, the published data are not conclusive, and further investigation is required. Cannabis may be associated with xerostomia, may promote caries development, may negatively affect the periodontium and may increase the incidence of oral lesions. We used this information to develop a framework to guide clinicians in assessing and treating patients with a history of cannabis use.
Conclusions:
The survey findings suggest that cannabis use is on the rise in Canada, with implications for the provision of dental care. Cannabis may alter the effects of drugs used in the dental setting and may be associated with an increased incidence of oral health issues. The dental management strategies suggested here are intended as an informative reference for clinicians. Additional research is needed to further elucidate the long-term effects of cannabis on oral health, its drug interactions and its potential medicinal applications in dentistry.
Introduction
Cannabis, known colloquially as marijuana or “weed,” has received increasing attention in recent years, both for its evolving legal status in numerous countries and its potential impact on public health.1 Currently, cannabis has been legalized for recreational use in 24 of the 50 US states and for medical use in 38 states.2 In Canada, cannabis was legalized in October 2018 through the Cannabis Act, which provides a comprehensive framework for its regulation.3,4 Cannabis originates primarily from 3 species: Cannabis sativa, Cannabis indica and Cannabis ruderalis, all of which have been cultivated for at least 6000 years.5 Cannabis contains more than 400 chemical compounds, with the 2 most investigated constituents being tetrahydrocannabinol (THC) and cannabidiol (CBD).6 Cannabis can be consumed through smoking, vaporization, oral ingestion and intravenously, each of which exposes the oral tissues to cannabinoids in distinct ways.7 With increasing acceptance and accessibility of cannabis, health care providers, including oral health professionals, are more frequently encountering patients with a history of cannabis use. This trend highlights the need for evidence-based guidelines on how cannabis use affects oral health and its management in dental care. Studies suggest that cannabis use is associated with various oral health concerns, including xerostomia, increased caries risk, periodontal disease and potential links to oral malignancies. Despite these emerging concerns, there remains a gap in knowledge regarding the impact of cannabis on oral health and how dental practitioners should manage the care of patients with a history of cannabis use.
This review aims to examine the interplay between cannabis use and oral health, providing an overview of its pharmacology, potential implications and key considerations for the dental management of patients with a history of cannabis use. We begin by providing an update on consumption trends in Canada since 2017. Next, we provide a summary of cannabis pharmacology and discuss potential interactions of cannabis with drugs commonly used in the dental setting. We then examine the effects of cannabis on caries, the periodontium and oral cancer. Finally, we propose a sequence of steps for and provide information on the dental management of patients with a history of cannabis use. By addressing these topics, we aim to educate dental professionals and contribute to the development of informed clinical practices for managing patients who use cannabis.
Methods
We compiled data on self-reported cannabis use from the Canadian Cannabis Survey (CCS), distributed by Health Canada, to evaluate cannabis consumption trends across the country.8 First distributed in 2017, the CCS collects detailed data on cannabis consumption, pricing and other parameters. For each iteration of this annual survey, participants were selected randomly, were contacted by phone and were screened to determine eligibility. Eligible candidates who agreed to participate received a link to the online survey. Data were collected from individuals 16 years of age or older across all Canadian provinces and territories. For our analysis, we focused on data for national nonmedical cannabis use from 2017 to 2024, reported on an annual basis, in participants with a minimum age of 16 years. Although survey-based statistics were reported by the CCS,8 the specific statistical tests were not described; therefore, we performed statistical analysis using a 2-tailed z-test with a significance level of p < 0.01.
For the scoping review, we performed a detailed search for relevant articles using PubMed, Google Scholar and the University of Toronto Library database, which also includes the JSTOR, OVID, Scopus and Web of Science databases. Search terms included “cannabis” or “marijuana” and “pharmacology” or “mechanism of action,” “drug interactions,” “oral health” or “mouth” or “oral cavity,” “management” or “dental management,” and “acute effects.” We also performed more specific searches using the terms “cannabis” or “marijuana” and “analgesics,” “sedatives,” “xerostomia,” “caries,” or “oral cancer.” We included electronic peer-reviewed manuscripts, reviews and/or book chapters, preferably published in 2019 or later and written in or translated into English. Where information on a particular topic was limited, older publications were used. Guidelines from regulatory bodies were also included. Articles that were not peer-reviewed, those not accessible electronically, editorials and letters, and those not available in English were excluded. These criteria were intended to provide a current, evidence-based perspective on the impact of cannabis on oral health. The most recent search was performed in October 2024, and over 65% of the included references were published in 2019 or later. The articles examined in this review were classified according to their primary focus on the topics of interest, with some covering more than one topic area.
Results
Cannabis Consumption before and after Legalization in Canada
To evaluate cannabis consumption trends across Canada, we extracted cannabis use trends from the CCS.8 From 2017 to 2024, an overall upward trend was observed in the proportion of Canadians who reported consuming cannabis (Fig. 1). Cannabis was legalized in 2018, and the CCS data showed a significant increase (by 3.8 percentage points) in cannabis use from 2018 to 2024 (p < 0.001). The 2022 iteration of the CCS survey8 determined that stress, anxiety, boredom, loneliness and lack of a regular schedule were the most common reasons for increased cannabis consumption.
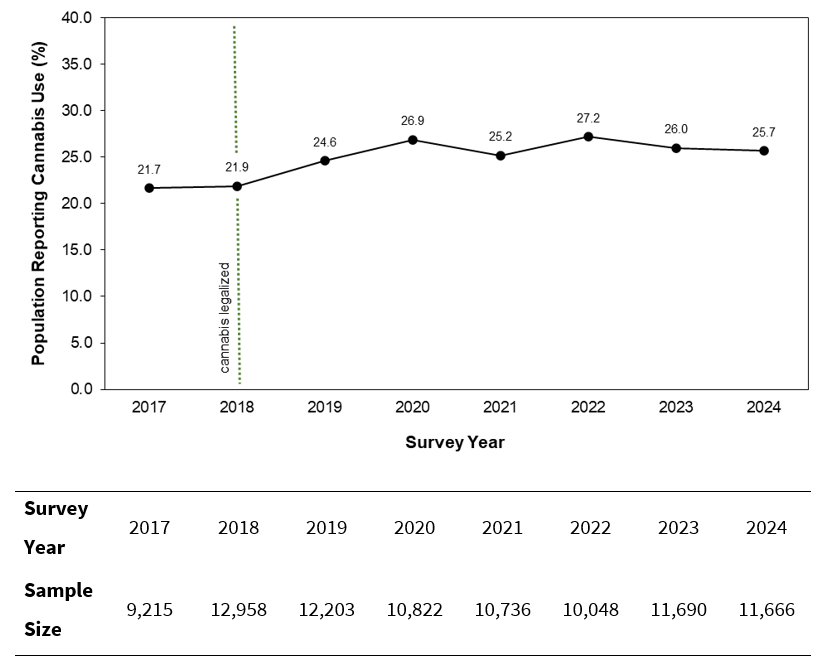
Figure 1: Proportion of the Canadian population self-reporting cannabis use, 2017 to 2024, based on the Canadian Cannabis Survey. After 2018, when cannabis was legalized in Canada (dotted green line), there was a significant increase in self-reported cannabis use (p < 0.001; 2-tailed z-test).
To determine the potential impact of COVID-19, the CCS surveys for 2020 to 2022 asked participants whether their cannabis use had changed as a result of the pandemic.8 In all 3 of these survey years, more than 50% of respondents reported that they had maintained the same level of cannabis use, and less than 25% indicated an increase in cannabis use. However, across all 3 years, increases in cannabis consumption were primarily observed in younger age groups (16–19 years and 20–24 years). As cannabis use rises across the country, clinicians should routinely seek out and review updates on the impact of cannabis on oral health, monitor and record patients’ history of use, advise patients on the effects of cannabis and treat them as outlined in this review.
Cannabis Pharmacology
Cannabis exerts its effects within the human body by targeting the endocannabinoid system,9,10 a complex network of receptors, endocannabinoids and enzymes that play vital roles in various physiologic processes, including the maintenance of homeostasis throughout the body. THC and CBD, 2 of the most abundantly produced and widely studied constituents of C. sativa, interact with the endocannabinoid system through the cannabinoid receptors.10 These G-protein–coupled receptors are divided into cannabinoid receptor type 1 (CB1) and cannabinoid receptor type 2 (CB2).11-13 CB1 is found predominantly in the central nervous system, particularly in the basal ganglia, cerebellum, frontal cortex, hippocampus and spinal cord, as well as the peripheral nervous system.11,12 CB2 is more abundant in the immune system, glia cells and hematopoietic cells. Although CB2 is typically expressed in the periphery, unhealthy states can lead to upregulation of CB2 in the brain.14 Both CB1 and CB2 are also expressed in the cardiovascular system. In the oral cavity, the cannabinoid receptors are expressed in periodontal tissues, salivary glands and periodontal tissues.15
THC is a partial agonist of CB1 and CB2.10 By targeting CB1, THC exerts psychoactive, analgesic and antiemetic effects, as well as relieving muscle spasticity and pain, increasing appetite and protecting neurons from damage induced by the neurotransmitter glutamate (Fig. 2). Through modulation of CB2, THC improves neurologic function and reduces inflammation. Conversely, CBD is a negative allosteric modulator of CB1 and an agonist of CB2 and has significantly lower affinity for the cannabinoid receptors relative to CB1. CBD modulates the emotional and cognitive aspects of pain perception through CB1 and contributes to pain perception and processing through CB2.10 These interactions may affect dental pain management, periodontal disease and oral mucosal health.
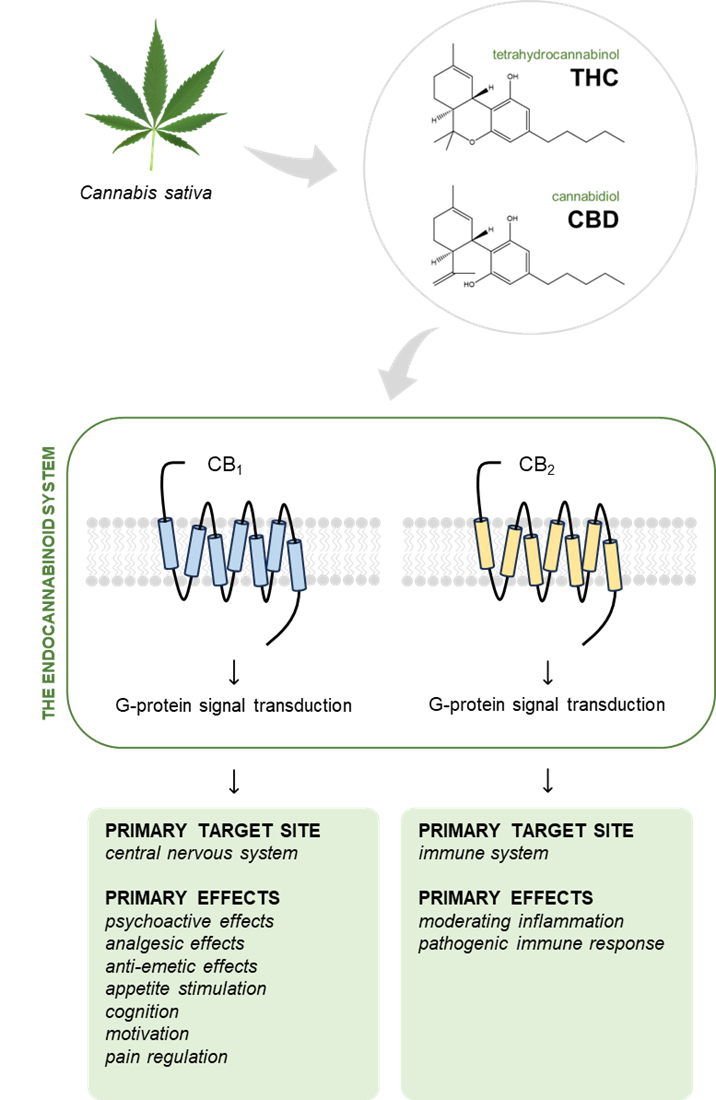
Figure 2: Tetrahydrocannabinol (THC) and cannabidiol (CBD), the most abundant molecules derived from the Cannabis sativa plant, target the cannabinoid receptor 1 (CB1) and cannabinoid receptor 2 (CB2) in the endocannabinoid system to varying degrees. Through these receptors, cannabinoids primarily exert their effects on both the central nervous system and the immune system. Despite the similarity of their chemical structures, THC and CBD have differing effects, as described above. (Cannabis sativa image used under the Creative Commons license from freepik.com)
Drug Interactions
To date, there has been limited research on drug interactions between cannabinoids and the drugs commonly associated with dental treatment. However, potential interactions should be considered when patients who use cannabis require dental treatment, because both THC and CBD primarily affect the cytochrome P450 (CYP) enzymes.16-18 Studies have shown that THC is primarily metabolized by CYP2C19 and CYP3A4, whereas CBD is primarily metabolized by CYP2C9, CYP3A4 and CYP2D6.17,18 The CYP3A4 isozyme, which metabolizes both THC and CBD, accounts for the metabolism of over 30% of drugs used today and is the most abundant cytochrome P450 isozyme in the human body.19 Some of the regularly used dental drugs that are metabolized by CYP3A4 are erythromycin, clarithromycin, midazolam, acetaminophen, lidocaine, mepivacaine, nystatin and certain benzodiazepines.16 Additionally, CYP2C9 and CYP2D6 have been shown to metabolize some nonsteroidal anti-inflammatory drugs (NSAIDs) and some opioids, respectively. Considering that CBD and THC can be metabolized by several enzymes, there is potential that they may increase the serum level of drugs used during dental treatment, which could potentially heighten their effects and risk adverse effects. Therefore, it is crucial for dentists to be vigilant in monitoring patients who use cannabis and to apply the monitoring strategies explained below.
Interactions with Analgesics
Dental pain may arise due to trauma, infection or treatment. To manage this pain, dentists frequently prescribe analgesics, including both non-opioid options, such as acetaminophen and NSAIDs, and opioids.20 To date, there is limited literature on drug interactions between cannabinoids and these common analgesics. Some studies have suggested that when acetaminophen is taken along with CBD, there can be adverse effects on the liver.21 Acetaminophen is metabolized in the liver through glucuronidation, sulfation and oxidation.22 Glucuronidation, the main biotransformation pathway of acetaminophen, is carried out by uridine 5'-diphospho-glucuronosyltransferases (UGTs), which are phase II drug-metabolizing enzymes.22,23 This group includes UGT1A1, UGT1A6, UGT1A9 and UGT2B15, which are responsible for converting acetaminophen into its nontoxic forms, which can be excreted from the body.21 However, a small portion of acetaminophen is oxidized into a reactive metabolite known as N-acetyl-benzoquinone (NAPQI), which can cause liver hepatoxicity when present in high amounts. When acetaminophen is consumed along with CBD, the CBD may inhibit the UGT enzymes, thus reducing the liver’s ability to metabolize acetaminophen through glucuronidation. As a result, more acetaminophen is redirected toward the formation of NAPQI, which increases the risk of liver hepatoxicity. This interaction suggests a potential risk when cannabis is combined with acetaminophen.
Reports of interactions of cannabis with NSAIDs are also limited in the literature. The UGT enzymes are reported to be involved in the metabolism and excretion of NSAIDs.24,25 In particular, ibuprofen is metabolized by UGT1A9 and UGT2B7, and naproxen is metabolized by UGT2B7.24 One study assessed the inhibition potential of CBD and THC.25 The greatest inhibition was seen with CBD against the glucuronidation activity of UGT1A9, UGT2B4, UGT1A6 and UGT2B7. In addition, strong inhibition of UGT1A9 and UGT2B7 was also demonstrated by THC and CBD. These results suggest that THC and CBD may inhibit the UGT enzymes, subsequently increasing the bioavailability of ibuprofen and naproxen.24,25 Although these studies all showed increased drug concentration in the blood, we found no studies reporting the clinical implications of these results. Future studies should aim to explore the significance of these findings, especially for patients with hepatic or kidney function.
Opioids, which are frequently prescribed by dentists after oral surgery, have also been found to interact with cannabinoids. One study explored potential interactions between morphine, an opioid analgesic that is structurally like oxycodone, and the major cannabinoids.26 Morphine is primarily metabolized by UGT2B7 into an inactive metabolite, morphine-3-glucuronomide (M3G), and an active metabolite, morphine-6-glucuronide (M6G). As previously mentioned, CBD and THC inhibit the major UGT enzymes, so the authors used in vitro assays and mechanistic modelling to explore whether cannabis or its major metabolites inhibit morphine glucuronidation by UGT2B7. They found that THC and CBD and their respective metabolites inhibited UGT2B7, with CBD and THC exhibiting the most potent inhibition. The inhibitory constant values for CBD and THC, evaluated against the formation of M3G and M6G, were similar to the concentration of cannabinoids found in the body after cannabis consumption. These results suggest that cannabinoids can inhibit morphine metabolism at concentrations typically seen in the body after cannabis use. This raises the possibility of drug interactions between cannabinoids and morphine. However, the literature remains unclear as to whether these findings carry any clinical significance. Future studies should explore the clinical impact of these interactions through a dynamic model.
Interactions with Anesthetic and Sedative Drugs
Dental treatment is often accompanied by the administration of anesthetics and sedatives to reduce or eliminate pain, alleviate anxiety and/or allow the performance of complex surgical procedures. Consequently, investigating interactions between cannabis and these compounds is valuable to clinical practice. As one example, Moran and colleagues27 found no significant differences in achievement of profound local anesthesia, in terms of either onset or duration of action, between patients with chronic cannabis use and those who did not use cannabis. All participants received standard maxillary infiltration of a lateral incisor with 2% lidocaine (1:100 000 epinephrine, 1.7 mL).
Some studies have suggested that epinephrine-containing local anesthetics may exacerbate the cardiac arrhythmias typically induced by cannabis metabolites due to sympathetic activity and parasympathetic inhibition.28,29 This sympathetic hyperactivity can amplify the cardiovascular response to surgical stress, increasing heart rate and contractility. After marijuana use, heart rate may rise by 20% and 100%, peaking 10 to 30 minutes after consumption.28,30 The high liposolubility of cannabis can extend these tachycardic effects for up to 72 hours.28 The initial response to cannabis consumption can be followed by increased parasympathetic tone, resulting in bradycardia and hypotension.28,31 Theoretically these cardiovascular effects may present a concern for dental patients. However, there is currently limited evidence concerning drug interactions between cannabis and local anesthetics containing epinephrine.32
General anesthesia and inhaled anesthetics are also associated with complications in patients with routine cannabis use.33-37 One clinical study examined the postoperative cardiovascular effects of CBD following surgical removal of third molars in healthy patients.33 Patients who received general anesthesia within 72 hours of smoking cannabis (but not patients who did not smoke cannabis) had sustained and abnormal tachycardia for 38 minutes postoperatively, with return to baseline sinus rhythm after 19 minutes. The authors explained that this effect may have been caused by a sympathoadrenal response to CBD mediated by the central nervous system. Similarly, in a retrospective clinical study by Twardowski and colleagues,34 patients with a history of chronic cannabis use required an additional 14% fentanyl, 19.6% midazolam and 220.5% propofol, relative to nonusers, to maintain anesthesia for the duration of endoscopy. Another retrospective study reported increased tolerance to the inhaled anesthetic sevoflurane in patients who used cannabis.35 Other studies have found that cannabis users are at increased risk of stroke and postoperative myocardial infarction.36,37 Together, these studies suggest that chronic cannabis use combined with anesthesia may be associated with an increased risk of complications. Clinicians should be prudent when administering anesthesia to patients with chronic cannabis use and should closely monitor them postoperatively.
Effect of Cannabis on Oral Health
Cannabis, Xerostomia and Caries
Some studies have reported that cannabis users generally experience dry mouth or xerostomia after using cannabis.38,39 In one study, 70% of cannabis smokers had symptoms of xerostomia, with these effects starting immediately after the use of cannabis and lasting between 1 and 6 hours.38 In another study, occasional dry mouth was observed in 67.2% of adolescents who used cannabis on 6 days or more in the previous 30 days, with frequent or ongoing dry mouth observed in 19.4% .39 These effects were significantly higher in the cannabis-smoking group than in a comparison group of nonusers (p < 0.003).
Additionally, cannabis consumption has been found to influence leptin, an important hormone for regulating appetite.40 Consequently, following the consumption of cannabis, users often feel hungry. In a survey study carried out by Schulz-Katterbach and colleagues,38 63% of participants who reported feeling hungry after cannabis use consumed food and drinks that were categorized as being sweet. Another recent study also found frequent consumption of sugary snacks and beverages among cannabis users.41 The combination of xerostomia and cariogenic diet after the consumption of cannabis can leave teeth vulnerable to attacks from potentially cariogenic food and drinks, increasing a patient’s caries risk.40 Table 1 and Fig. 3 highlight reported associations between cannabis and oral disease.
|
Topic |
Articles |
Key findings |
|---|---|---|
| Xerostomia | Chaffee et al. (2023)39 Schulz-Katterbach et al. (2009)38 |
|
| Caries | Joshi and Ashley (2016)40 Le et al.(2022)41 Schulz-Katterbach et al. (2009)38 |
|
| Periodontal disease | Chaffee et al. (2023)39 Joshi and Ashley (2016)40 Le et al. (2022)41 Martinez-Garcia and Hernández-Lemus (2021)43 Scott et al. (2022)42 Shariff et al. (2017)44 Thomson et al. (2008)46 |
|
| Oral cancer | Cretu et al. (2024)51 Gambhir et al. (2012)47 Huang et al. (2023)50 Joshi and Ashley (2016)40 Le et al. (2022, 2019)41,48 Warnakulasuriya (2009)49 |
|
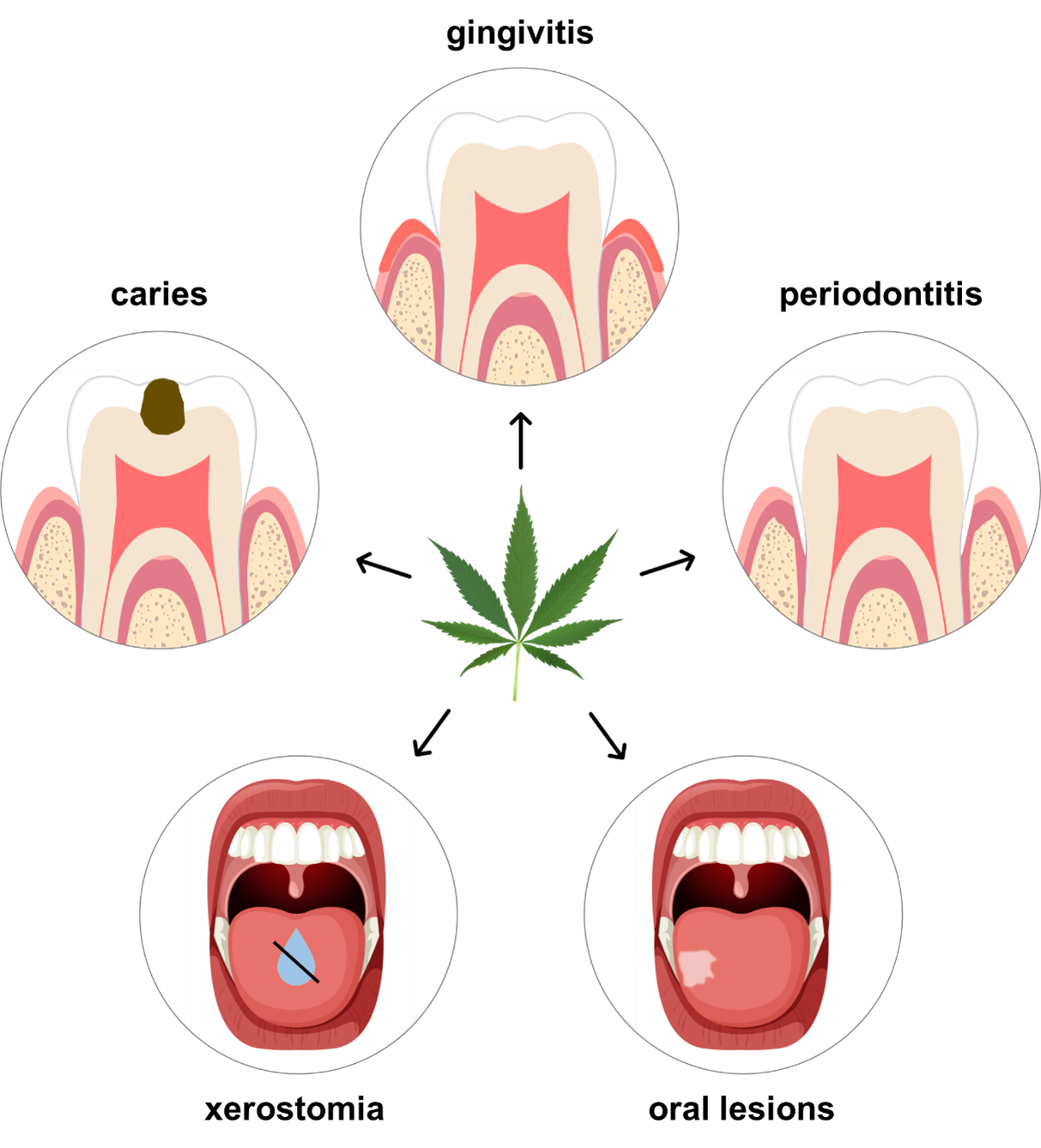
Figure 3: Potential impact of cannabis on dental disease. Data suggest that cannabis is associated with xerostomia, caries, gingivitis, periodontitis, and oral lesions. (Cannabis sativa image used under the Creative Commons license; tooth and mouth images adapted and used with permission from freepik.com.)
Cannabis and the Periodontium
Cannabis users frequently experience compromised periodontal health, primarily attributed to factors such as reduced saliva production, irregular oral hygiene practices and consumption of cariogenic food.40-42 All of these factors contribute to the accumulation of biofilm and plaque, which can trigger an inflammatory process called gingivitis. Over time, if oral hygiene is not improved and the plaque is left to harden into calculus, bacterial toxins are produced, which trigger a more severe inflammatory response called periodontitis. During periodontitis, the inflammation extends deeper into the surrounding structures of the teeth, including the periodontal ligament and the underlying bone, irreversibly damaging the patient’s periodontium.40,43 Shariff and colleagues44 found that individuals who use cannabis frequently exhibited a greater prevalence of sites with pocket depths exceeding 4 mm and clinical attachment loss, relative to those who did not use cannabis. Additionally, they observed that periodontitis might manifest at an earlier stage among cannabis users than among nonusers.
Chaffee45 and Thomson and colleagues46 reported that cannabis use during adolescence and young adulthood was associated with worsening periodontal condition over the next decade of life, even when the data were controlled for tobacco smoking, socioeconomic status, irregular dental services and dental plaque. European study participants were categorized as having no exposure (293 individuals), some exposure (up to 40 instances of cannabis use in the previous year, 428 individuals) or high exposure (> 40 instances of cannabis use in the previous year, 182 individuals) to cannabis, with follow-up at 18, 21, 26 and 32 years of age. 46 Individuals in the high exposure category were determined to have a relative risks of 2.2 (95% confidence interval [CI] 1.2–3.9) for incident clinical attachment loss (CAL) relative to the group with no exposure, 1.6 (95% CI 1.2–2.2) for CAL of 4 mm or greater in at least one site, and 3.1 (95% CI 1.5–6.4) for CAL of 5 mm or greater in at least one site, even when the data were controlled for tobacco use, plaque, infrequent dental visits and sex.
Cannabis and Oral Cancer
Although the correlation between tobacco use and oral cancer has been extensively documented, the literature remains unclear as to whether cannabis is a risk factor for oral cancer.47 Cannabis smoke contains twice the number of carcinogens as tobacco smoke, the most potent compounds being phenols, nitrosamines, vinyl chloride and various polycyclic aromatic hydrocarbons.40 Through chronic use of cannabis, these carcinogens can damage the cells in the oral cavity and increase the risk of oral leukoplakia (thick white patches on the oral mucosa), erythroplakia (thick red patches without an obvious cause) and premalignant lesions.41,47,48 As a result, cannabis users may be at higher risk of oral cancers; some studies have also reported an increased risk of head and neck cancer with cannabis consumption.48 Overall, however, there is inconclusive evidence to implicate cannabis as a cause of oral cancer, and other studies have not found a significant association.48-51
Dental Management of Patients with a History of Cannabis Use
With the increase in cannabis use across Canada, dental practitioners will more frequently encounter patients with a history of cannabis use. Clinicians should routinely record detailed information on each patient’s drug use to monitor cannabis use and abuse. The Royal College of Dental Surgeons of Ontario (RCDSO) has developed a series of medical questions that dental clinicians can ask their patients when discussing cannabis.52 Although this questionnaire was created by the dental regulatory body in Ontario, it is relevant for practitioners across the entire country.
Dental staff will also need to be able to identify patients presenting to the clinic while under the influence of cannabis and record detailed information on the patient’s drug use to allow proper management in the clinical setting. The acute effects of cannabis use may be categorized into physical signs, subjective symptoms and psychiatric effects (Table 2).53 Clinicians and dental staff should monitor for these signs and symptoms, especially in patients with a known history of cannabis use. Physical signs will generally be easily detected either through observation or by measuring vital signs, whereas the subjective symptoms will vary among individuals. Psychiatric effects may occur more commonly in patients who use high doses of cannabis, particularly with intravenous administration.54 However, some studies have suggested that cannabis administered orally or through ingestion may lead to similar symptoms, regardless of dose.54,55
|
Physical signs |
Subjective symptoms |
Psychiatric effects |
|---|---|---|
| Conjunctival hyperemia Increased appetite Increased blood pressure Tachycardia Xerostomia |
Continuous laughter Continuous talkativeness Euphoria Lethargy Perceptual distortion Sedation Social withdrawal |
Anxiety Delusions Depersonalization Disordered thinking Hallucinations Impaired memory Irrational panic Reduced attention span Psychomotor agitation Psychosis |
One study examined the perfusion index of patients presenting to the emergency department following cannabis consumption.56 The authors found that among patients admitted to the hospital within 3 hours of cannabis consumption, average peripheral oxygen saturation was 92.8%, notably lower than the normal range of 95% to 100%. Affected patients commonly showed symptoms of dyspnea, agitation, sweating, palpitations and nausea. These findings suggest that pulse oximetry can play an important role in identifying patients who have recently consumed cannabis. Additionally, oximetry can help the dental clinician to assess organ profusion and monitor for tissue hypoxia. Therefore, we recommend that dental professionals use pulse oximetry for patients suspected of acute cannabis intoxication and those with a known history of cannabis use.56
On the basis of RCDSO recommendations52 and the literature summarized above, we propose a set of guidelines for dental management of patients with a history of cannabis use (Table 3). These guidelines are intended as an informative resource for dentists and dental auxiliary staff to become more comfortable with the rise in cannabis use that has occurred since legalization in Canada. Because cannabis may impair judgement, the clinician must evaluate patients’ ability to provide informed consent. Dentists also have a duty to ensure that patients who are found to be under the influence of cannabis at an appointment should not drive a vehicle or participate in activities that may harm themselves or others.
| 1. Record a comprehensive medical history, including the questions suggested by the Royal College of Dental Surgeons of Ontario.52 |
2. Observe the patient for signs of acute cannabis use (Table 2) or cannabis use disorder.
|
| 3. Examine the oral cavity for conditions associated with cannabis use (xerostomia, caries, gingivitis, periodontitis, oral cancer, other oral lesions). |
| 4. Educate the patient about the impact of routine cannabis use on oral health. Create a supportive environment for the patient to discuss their cannabis use and to learn about the impact of cannabis. |
| 5. Although known drug interactions with cannabis remain limited, use caution when prescribing medications or using nitrous oxide, local anesthetic, or general anesthetic in patients known to also use cannabis. Monitor and record any adverse reactions. |
Discussion
In this review, we have reported on Canada-wide trends in the consumption of cannabis from 2017 to 2024. Since cannabis legalization in 2018, the proportion of self-reported cannabis use has increased significantly, by 3.8 percentage points, with stress, anxiety, boredom and loneliness being cited as key reasons for increased consumption. Additionally, during the COVID-19 pandemic (2020–2022), most users maintained their consumption levels, with less than 25% reporting increased consumption, primarily those aged 16 to 24 years. With these changes in cannabis use, dental professionals should stay up to date with effects on oral health and patient care.
To guide clinicians, we reviewed the literature to garner insight into cannabis pharmacology and its interactions with commonly used drugs relevant to dentistry, including analgesics, sedatives and general anesthetic agents. Although cannabinoids may theoretically interact with these drugs, limited evidence exists on direct associations between cannabis and analgesics, including acetaminophen, NSAIDs and opioids, as well as local anesthetics. Some studies have reported that cannabis users had increased tolerance to inhaled anesthetics, required higher doses of general anesthetics, and experienced more intraoperative and postoperative complications. However, the existing literature is inconclusive, and additional research is needed to further investigate cannabis–drug interactions.
We also explored the effects of cannabis consumption on oral health. Specifically, we found that cannabis may be associated with xerostomia, promote caries development, negatively affect the periodontium and increase the incidence of oral lesions. Through our literature search, we found direct evidence for associations between cannabis use and xerostomia and periodontal disease. Notably, although smoking remains the predominant method of cannabis consumption, an increasing number of people are consuming cannabis as edible products or through vaporizers. To date, no published studies have investigated the various delivery methods and their respective effects on the periodontium. Thus, as cannabis consumption continues to become more popular and as alternative routes of administration arise, more research is needed to understand the effects on oral health and how to effectively manage these patients. Additionally, while cannabis is associated with hyposalivation and an increased cariogenic diet, the evidence for an association between cannabis and caries is not as robust. Furthermore, although cannabis has been linked to an increased incidence of oral lesions, the evidence relating to risk of oral cancer remains inconclusive. Further research is needed to elucidate the long-term impact of cannabis use on oral health.
As cannabis becomes more accessible in various formats across Canada and other parts of the world, it is critical for clinicians to be equipped with the knowledge and resources necessary to manage the oral effects of this substance. Therefore, in this review we have introduced a questionnaire adapted from the RCDSO to medically screen patients for cannabis use, provided the signs and symptoms to assess acute effects of cannabis intoxication, and outlined a set of guidelines for the dental management of patients with a history of cannabis use. We hope that clinicians will incorporate this knowledge into their practices to educate and treat patients accordingly.
Despite our summary of the effects of cannabis on oral health, there are many areas requiring further investigation and research. Cannabis can be consumed by several means, including inhalation and ingestion, yet most of the available research focuses on inhalation. As a result, our findings cannot be generalized to all modes of consumption, and the potential differences in oral health outcomes based on route of administration remain unclear. Additionally, while the pharmacology of cannabis is well established, drug interactions and oral health impacts are not as well known. Many of the reported drug interactions with cannabis are theoretical, based on mechanism of action, rather than being well established from clinical settings. It also remains unknown whether the oral health effects of cannabis are dose-dependent or if they are reversible upon cessation. Furthermore, the use of medicinal cannabis may also have applications for dentistry, but this aspect was not explored here. With the continued consumption of cannabis in Canada, the magnitude of its impact on oral health will become more evident. For now, dentists should caution their patients about suspected risks associated with cannabis use and monitor their patients to limit the effects of cannabis use on patient care.
Conclusion
In this review, we have provided a comprehensive overview of cannabis and its implications for oral health, noting the documented increase in cannabis use since legalization in Canada. We have provided a summary of cannabis pharmacology and detailed potential interactions with drugs commonly used in dentistry. We have also demonstrated the effects that cannabis may have on oral health. Finally, we have suggested a sequence of steps to help dental practitioners manage the care of patients with a history of cannabis use. Moving forward, future research should further investigate the impact of cannabis on dental disease and the interactions of this substance with commonly used dental drugs, and should develop a greater understanding of the medicinal role that cannabis can play during dental treatment. Overall, we anticipate that this review will serve as a valuable reference for dentists in clinical practice.
THE AUTHORS
Corresponding author: Dr. Aviv Ouanounou, Department of Clinical Sciences, Pharmacology and Preventive Dentistry, Faculty of Dentistry, University of Toronto, 124 Edward Street, Room 370, Toronto, ON M5G 1G6. Email: aviv.ouanounou@dentistry.utoronto.ca
The authors have no declared financial interests.
This article has been peer reviewed.
References
- Smart R, Pacula RL. Early evidence of the impact of cannabis legalization on cannabis use, cannabis use disorder, and the use of other substances: Findings from state policy evaluations. Am J Drug Alcohol Abuse. 2019;45(6):644-63. doi: 10.1080/00952990.2019.1669626
- Baldwin GT, Vivolo-Kantor A, Hoots B, Roehler DR, Ko JY. Current cannabis use in the United States: Implications for public health research. Am J Public Health. 2024;114(S8):S624-7. doi: 10.2105/AJPH.2024.307823
- Cannabis Act, S.C. 2018, c. 16. Available: https://laws-lois.justice.gc.ca/eng/acts/c-24.5/FullText.html (accessed 2024 Nov 4).
- Hamid MA, Shaikh R, Gunaseelan L, Salim J, Arulchelvan A, Tulloch T. Recreational cannabis legalization in Canada: A pediatrics perspective. Subst Use Misuse. 2022;57(3):481-3. doi: 10.1080/10826084.2021.2012689
- Li HL. An archaeological and historical account of cannabis in China. Econ Bot. 1973;28(4):437-48. doi: 10.1007/BF02862859/
- Atakan Z. Cannabis, a complex plant: different compounds and different effects on individuals. Ther Adv Psychopharmacol. 2012;2(6):241-54. doi: 10.1177/2045125312457586
- Vinette B, Cote J, El-Akhras A, Mrad H, Chicoine G, Bilodeau K. Routes of administration, reasons for use, and approved indications of medical cannabis in oncology: a scoping review. BMC Cancer. 2022;22:319. doi: 10.1186/S12885-022-09378-7
- Cannabis use for non-medical purposes among Canadians (aged 16+). Ottawa (ON): Government of Canada; [December 2024]. Available: https://health-infobase.canada.ca/cannabis/.
- Ronan PJ, Wongngamnit N, Beresford TP. Molecular mechanisms of cannabis signaling in the brain. Prog Mol Biol Transl Sci. 2016;137:123-47. doi: 10.1016/bs.pmbts.2015.10.002
- Jurga M, Jurga A, Jurga K, Kazmierczak B, Kusmierczyk K, Chabowski M. Cannabis-based phytocannabinoids: Overview, mechanism of action, therapeutic application, production, and affecting environmental factors. Int J Mol Sci. 2024;25(20):11258. doi: 10.3390/ijms252011258
- Ebbert JO, Scharf EL, Hurt RT. Medical cannabis. Mayo Clin Proc. 2018;93(12):1842-7. doi: 10.1016/j.mayocp.2018.09.005
- Adel Y, Alexander SPH. Neuromolecular mechanisms of cannabis action. In: Murillo-Rodriguez E, Pandi-Perumal SR, Monti JM, editors. Cannabinoids and neuropsychiatric disorders. Adv Exp Med Biol. 2021;1264:15-28. doi: 10.1007/978-3-030-57369-0_2
- Lu HC, Mackie K. Review of the endocannabinoid system. Biol Psychiatry Cogn Neurosci Neuroimaging. 2021;6(6):607-15. doi: 10.1016/j.bpsc.2020.07.016
- Chayasirisobhon S. Mechanisms of action and pharmacokinetics of cannabis. Perm J. 2021;25(1). doi: 10.7812/TPP/19.200
- Bellocchio L, Inchingolo AD, Inchingolo AM, Lorusso F, Malcangi G, Santacroce L, et al. Cannabinoids drugs and oral health—from recreational side-effects to medicinal purposes: A systematic review. Int J Mol Sci. 2021;22(15):8329. doi: 10.3390/ijms22158329
- Abidi AH, Alghamdi SS, Derefinko K. A critical review of cannabis in medicine and dentistry: A look back and the path forward. Clin Exp Dent Res. 2022;8(3):613-31. doi: 10.1002/cre2.564
- Stout SM, Cimino NM. Exogenous cannabinoids as substrates, inhibitors, and inducers of human drug metabolizing enzymes: A systematic review. Drug Metab Rev. 2014;46(1):86-95. doi: 10.3109/03602532.2013.849268
- Beers JL, Fu D, Jackson KD. Cytochrome P450–catalyzed metabolism of cannabidiol to the active metabolite 7-hydroxy-cannabidiol. Drug Metab Dispos. 2021;49(10):882-91. doi: 10.1124/dmd.120.000350
- Zhao M, Ma J, Li M, Zhang Y, Jiang B, Zhao X, et al. Cytochrome P450 enzymes and drug metabolism in humans. Int J Mol Sci. 2021;22(23):12808. doi: 10.3390/ijms222312808
- Moussa N, Ogle OE. Acute pain management. Oral Maxillofac Surg Clin North Am. 2022;34(1):35-47. doi: 10.1016/j.coms.2021.08.014
- Vazquez M, Guevara N, Maldonado C, Guido PC, Schaiquevich P. Potential pharmacokinetic drug-drug interactions between cannabinoids and drugs used for chronic pain. Biomed Res Int. 2020;2020:3902740. doi: 10.1155/2020/3902740
- Mazaleuskaya LL, Sangkuhl K, Thorn CF, Fitzgerald GA, Altman RB, Klein TE. PharmGKB summary: Pathways of acetaminophen metabolism at the therapeutic versus toxic doses. Pharmacogenet Genomics. 2015;25(8):416-26. doi: 10.1097/FPC.0000000000000150
- Ewing LE, McGill MR, Yee EU, Quick CM, Skinner CM, Kennon-McGill S, et al. Paradoxical patterns of sinusoidal obstruction syndrome-like liver injury in aged female CD-1 mice triggered by cannabidiol-rich cannabis extract and acetaminophen co-administration. Molecules. 2019;24(12):2256. doi: 10.3390/molecules24122256
- Brown JD, Winterstein AG. Potential adverse drug events and drug–drug interactions with medical and consumer cannabidiol (CBD) use. J Clin Med. 2019;8(7):989. doi: 10.3390/jcm8070989
- Nasrin S, Watson CJW, Bardhi K, Fort G, Chen G, Lazarus P. Inhibition of UDP-glucuronosyltransferase enzymes by major cannabinoids and their metabolites. Drug Metabol Dispos. 2021;49(12):1081-9. doi: 10.1124/dmd.121.000530
- Coates S, Bardhi K, Lazarus P. Cannabinoid-induced inhibition of morphine glucuronidation and the potential for in vivo drug–drug interactions. Pharmaceutics. 2024;16(3):418. doi: 10.3390/pharmaceutics16030418
- Moran MC, Heaton LJ, Leroux BG, Flake NM. Local anesthetic efficacy in marijuana users and nonusers: A pilot study. Anesth Prog. 2022;69(4):15-21. doi: 10.2344/anpr-69-02-08
- Echeverria-Villalobos M, Guevara Y, Mitchell J, Ryskamp D, Conner J, Bush M, et al. Potential perioperative cardiovascular outcomes in cannabis/cannabinoid users. A call for caution. Front Cardiovasc Med. 2024;11. doi: 10.3389/fcvm.2024.1343549
- Richards JR. Mechanisms for the risk of acute coronary syndrome and arrhythmia associated with phytogenic and synthetic cannabinoid use. J Cardiovasc Pharmacol Ther. 2020;25(6):508-22. doi: 10.1177/1074248420935743
- Latif Z, Garg N. The impact of marijuana on the cardiovascular system: A review of the most common cardiovascular events associated with marijuana use. J Clin Med. 2020;9(6):1925. doi: 10.3390/jcm9061925
- Ladha KS, Manoo V, Virji AF, Hanlon JG, McLaren-Blades A, Goel A, et al. The impact of perioperative cannabis use: A narrative scoping review. Cannabis Cannabinoid Res. 2019;4(4):219-30. doi: 10.1089/can.2019.0054
- Donaldson M, Goodchild JH. Is there a potentially serious drug interaction between marijuana and local anesthetics containing a vasoconstrictor? Gen Dent. 2024;72(3):14-9. PMID: 38640000
- Gregg JM, Campbell RL, Levin KJ, Ghia J, Elliott RA. Cardiovascular effects of cannabinol during oral surgery. Anesth Analg. 1976;55(2):203-13. doi: 10.1213/00000539-197603000-00017
- Twardowski MA, Link MM, Twardowski NM. Effects of cannabis use on sedation requirements for endoscopic procedures. J Osteopath Med. 2019;119(5):307-11. doi: 10.7556/jaoa.2019.052
- Holmen IC, Beach JP, Kaizer AM, Gumidyala R. The association between preoperative cannabis use and intraoperative inhaled anesthetic consumption: A retrospective study. J Clin Anesth. 2020;67:109980. doi: 10.1016/j.jclinane.2020.109980
- Goel A, McGuinness B, Jivraj NK, Wijeysundera DN, Mittleman MA, Bateman BT, et al. Cannabis use disorder and perioperative outcomes in major elective surgeries: A retrospective cohort analysis. Anesthesiology. 2020;132(4):625-35. doi: 10.1097/ALN.0000000000003067
- Hemachandra D, McKetin R, Cherbuin N, Anstey KJ. Heavy cannabis users at elevated risk of stroke: evidence from a general population survey. Aust N Z J Public Health. 2016;40(3):226-30. doi: 10.1111/1753-6405.12477
- Schulz-Katterbach M, Imfeld T, Imfeld C. Cannabis and caries—does regular cannabis use increase the risk of caries in cigarette smokers? Schweiz Monatsschr Zahnmed. 2009;119(6):576-83. PMID: 20112637
- Chaffee BW, Halpern-Felsher B, Cheng J. E-cigarette, cannabis and combustible tobacco use: associations with xerostomia among California adolescents. Community Dent Oral Epidemiol. 2023;51(2):180-6. doi: 10.1111/cdoe.12721
- Joshi S, Ashley M. Cannabis: A joint problem for patients and the dental profession. Br Dent J. 2016;220(11):597-601. doi: 10.1038/sj.bdj.2016.416
- Le A, Khoo E, Palamar JJ. Associations between oral health and cannabis use among adolescents and young adults: Implications for orthodontists. Int J Environ Res Public Health. 2022;19(22):15261. doi: 10.3390/ijerph192215261
- Scott DA, Dukka H, Saxena D. Potential mechanisms underlying marijuana-associated periodontal tissue destruction. J Dent Res. 2022;101(2):133-42. doi: 10.1177/00220345211036072
- Martinez-Garcia M, Hernandez-Lemus E. Periodontal inflammation and systemic diseases: An overview. Front Physiol. 2021;12:709438. doi: 10.3389/fphys.2021.709438
- Shariff JA, Ahluwalia KP, Papapanou PN. Relationship between frequent recreational cannabis (marijuana and hashish) use and periodontitis in adults in the United States: National Health and Nutrition Examination Survey 2011 to 2012. J Periodontol. 2017;88(3):273-80. doi: 10.1902/jop.2016.160370
- Chaffee BW. Cannabis use and oral health in a national cohort of adults. J Calif Dent Assoc. 2021;49(8):493-501. doi: 10.1080/19424396.2021.12222740
- Thomson WM, Poulton R, Broadbent JM, Moffitt TE, Caspi A, Beck JD, et al. Cannabis smoking and periodontal disease among young adults. JAMA. 2008;299(5):525-31. doi: 10.1001/jama.299.5.525
- Gambhir RS, Brar P, Anand S, Ranhawa A, Kakar H. Oral health aspects of cannabis use. Indian J Multidiscip Dent. 2012;2(3):507.
- Le A, Palamar JJ. Oral health implications of increased cannabis use among older adults: Another public health concern? J Subst Use. 2019;24(1):61-5. doi: 10.1080/14659891.2018.1508518
- Warnakulasuriya S. Causes of oral cancer – an appraisal of controversies. Br Dent J. 2009;207(10):471-5. doi: 10.1038/sj.bdj.2009.1009
- Huang P, Zhang PF, Li Q. Causal relationship between cannabis use and cancer: a genetically informed perspective. J Cancer Res Clin Oncol. 2023;149(11):8631-8. doi: 10.1007/s00432-023-04807-x
- Cretu B, Zamfir A, Bucurica S, Scheau AE, Savulescu Fiedler I, Caruntu C, et al. Role of cannabinoids in oral cancer. Int J Mol Sci. 2024;25(2):969. doi: 10.3390/ijms25020969
- Cannabis and dental health: what dentists should know. Royal College of Dental Surgeons of Ontario; 2019 Aug 23. Available: https://www.rcdso.org/en-ca/standards-guidelines-resources/rcdso-news/articles/5167 (accessed 2023 Sep 7).
- Karila L, Roux P, Rolland B, Benyamina A, Reynaud M, Aubin HJ, et al. Acute and long-term effects of cannabis use: a review. Curr Pharm Des. 2014;20(25):4112-8. doi: 10.2174/13816128113199990620
- Hindley G, Beck K, Borgan F, Ginestet CE, McCutcheon R, Kleinloog D, et al. Psychiatric symptoms caused by cannabis constituents: a systematic review and meta-analysis. Lancet Psychiatry. 2020;7(4):344-53. doi: 10.1016/S2215-0366(20)30074-2
- Bidwell LC, Karoly HC, Torres MO, Master A, Bryan AD, Hutchison KE. A naturalistic study of orally administered vs. inhaled legal market cannabis: cannabinoids exposure, intoxication, and impairment. Psychopharmacology (Berl). 2022;239(2):385-97. doi: 10.1007/S00213-021-06007-2
- Grigorasi GR, Corlade-Andrei M, Ciumanghel I, Sova I, Blaga T, Carp C, et al. Monitoring perfusion index in patients presenting to the emergency department due to drug use. J Pers Med. 2023;13(2):372. doi: 10.3390/jpm13020372



