Abstract
Introduction:
The Mandibular Function Impairment Questionnaire (MFIQ) was developed for clinical assessment of functional impairment in patients with temporomandibular disorder (TMD). It allows patients to rank difficulty performing 17 jaw-related functions as low, moderate or severe. Our study was designed to determine whether the MFIQ is also helpful in differentiating TMD from other causes of orofacial pain in a clinical setting.
Methods
A retrospective study was conducted at a private oral medicine/orofacial pain clinic. New patients who attended with orofacial pain complaints were selected (n = 174). All patients had filled out the MFIQ as part of new patient intake forms.
Results:
The study population consisted of 120 TMD patients, 25 patients with burning mouth syndrome (BMS), 19 with oral lesions (OLs) and 10 with trigeminal neuralgia (TN). TMD patients had significantly greater difficulty taking a large bite, yawning, chewing hard and resistant foods including meat, raw carrot, and apples compared with OL and BMS patients. The MFIQ alone was not able to distinguish between TMD and TN.
Conclusions:
The MFIQ is a short questionnaire that is openly accessible and can be completed relatively quickly by patients in a general dentistry clinic. High ranking of difficulty for items associated with taking a large bite, chewing hard or resistant food and yawning in the presence of complaint of orofacial pain should raise suspicion of TMD and TN as a possible source of the pain.
Introduction
The International Classification of Orofacial Pain defines facial pain as pain occurring under the orbitomeatal line, anterior to the pinnae and above the neck; it classifies facial pain as dentoalveolar, myofascial, temporomandibular joint (TMJ), neuropathic, headache-like pain, idiopathic or psychosocial. The most common cause of facial pain is reported to be myalgia related to temporomandibular disorder (TMD).1-2
Approximately 4% of the general population will complain of the onset of annoying to intense jaw pain annually, with 49% of those cases persisting more than 6 months. Facial pain occurs early in the course of TMD, even in those who never develop clinical signs of TMJ,3 which can manifest as joint noises, including clicking/crepitus with movement, deviation on opening and restricted movement. These signs can indicate alteration in joint anatomy, including internal derangement and/or degenerative bony changes in the mandibular condyles or glenoid fossa/articular eminence, which may not correlate clinically with functional impairment or interfere with daily function.4 TMD patients also commonly describe ear pain, headaches, neck pain, toothache, muscle tightness and tinnitus.5
Because the trigeminal nerve (i.e., cranial nerve V) innervates both sensory and motor function of the head and face region, the pain referral pattern from orofacial conditions — dental, headache or neuralgic — can mimic pain associated with TMD, leading patients to seek help from their dental provider.2 More serious conditions, such as osteomyelitis, temporal arteritis and neoplastic disorder, can also mimic TMD, making differentiation of TMD from other causes of orofacial pain especially important when prompt and proper care is paramount.6
The Mandibular Function Impairment Questionnaire (MFIQ) was created to aid assessment of jaw function impairment in patients with TMD by asking them to rank difficulty in performing 17 jaw functions.4 As clinical history alone cannot always differentiate between TMD-associated pain and referred pain that can mimic TMD pain, we looked at whether incorporating the short MFIQ into the initial assessment could help with diagnosis of TMD. We theorized that TMD patients would score higher on the MFIQ compared with patients with pain referred from a different origin.
Methods
A retrospective study was conducted at a private oral medicine and orofacial pain specialty clinic. All patient charts were reviewed for new patients who attended the clinic between October 2020 and April 2021. In October 2020, when virtual consultation was taking place, the MFIQ was added to the intake form to determine whether it could help diagnose TMD, based on consultation only. For many of our patients, additional tests and imaging studies, including hospital MRI, were requested to confirm diagnosis. This sometimes took up to a year. Thus, at the time of data collection in late 2021, we looked at the previous year to ensure that all patients included in the study had received a diagnosis supported by testing. We did not have research ethics board approval to review additional charts, so data collection was not extended.
All patients were assessed by the same clinician (MG). Diagnosis was obtained by clinical examination, follow-up laboratory and/or imaging studies and response to treatment. The MFIQ was not used to aid the diagnosis. We included all new patients who attended the clinic during the study period, had signed a form allowing their data to be used anonymously for a retrospective study, had completed the MFIQ and had received a diagnosis. Patients were excluded if they reported a recent motor vehicle accident and experienced pain in multiple areas in addition to orofacial pain, had not completed an MFIQ form, had not received a diagnosis or did not sign an authorization form.
Demographic data and data from the MFIQ were collected. Diagnoses were recorded based on imaging results and response to treatment at follow-up appointments. Patients were grouped by diagnosis: TMD, trigeminal neuralgia (TN), oral mucosal pain without intraoral lesions (burning mouth syndrome, BMS) or oral lesions/oral mucosal inflammation including aphthous ulcers, lichen planus and contact sensitive reactions (OL).
TMD
TMD was diagnosed when clinical examination revealed decreased maximum vertical opening, pain on palpation of the TMJ and/or associated muscles of mastication, deviation on opening or the presence of joint noises on jaw movement. Diagnosis was confirmed by MRI showing internal derangement and degenerative changes in 1 or both joints, as well as by response to medications such as anti-inflammatories and muscle relaxants, bruxism appliances and range of motion exercises.7-8
TN
TN was diagnosed when the patient had a history of episodic sharp shooting pain, and the pain was reproduced on palpation of trigger points. Diagnosis was confirmed by response to medication, central MRI protocol for TN and MRI of the TMJ, as well as follow-up assessment by neurology. MRI was used for these patients because frequent pain on palpation of the facial area made clinical differentiation difficult in some cases.9-10
Oral Lesions and BMS
For patients complaining of soreness, burning, dryness or taste changes, intraoral examination was done to look for lesions. If lesions were present, diagnosis was based on clinical presentation as well as response to treatment, including topical steroids.11-12
Patients with a normal intraoral examination were assessed for BMS with spatial taste testing, salivary flow testing, blood tests and imaging studies, including MRI and CT scan of the floor of the mouth as needed to rule out structural changes. BMS was diagnosed if patients had decreased unstimulated salivary flow and normal stimulated flow, demonstrated taste loss/confusion on spatial taste testing and on follow-up of their response to medication, including clonazepam, amitriptyline, gabapentin and/or pregabalin, as well as negative blood tests and imaging studies.13-15
MFIQ
The MFIQ consists of 17 items that patients rate on a 5-point Likert scale in terms of how difficult each item is to perform: 0 = no difficulty, 1 = a little difficulty, 2 = quite a bit of difficulty, 3 = much difficulty, 4 = very difficult or impossible without help.4 Ten items on the questionnaire assess function related to mastication; 5 assess daily activities, including speech, socialization and working; 2 items assess daily function not related to mastication, including yawning and kissing.
The sum of the ratings was used to calculate a raw component score, which was used to determine the level of functional impairment on a scale of 0 to 5. The qualitative level of functional impairment (MFIQ global score) was then determined as low (I), moderate (II) or severe (III) (Table 1).
|
Item scores (i), rated 0–4 on a Likert scale |
C = S/4N |
FIRS |
MFIQ score |
|---|---|---|---|
|
Note: C = raw component score, N = number of items rated, S = sum of ratings.4 |
|||
| All i < 2 | ≤ 0.3 | 0 | I, low |
| At least 1 i ≥ 2 | ≤ 0.3 | 2 | I, low |
| All i < 3 | 0.3–0.6 | 2 | II, moderate |
| At least 1 i ≥ 3 | 0.3–0.6 | 3 | II, moderate |
| All i ≠ 4 | > 0.6 | 4 | III, severe |
| At least 1 i = 4 | > 0.6 | 5 | III, severe |
Data Analysis
Data were analyzed using IBM SPSS with Χ2 test, Kruskal-Wallis test and Dunn-Bonferroni post-hoc testing with a significance level of 0.05.
Ethics Approval
This study was approved by the William Osler Health System Research Ethics Board.
Results
The study population consisted of 174 patients: 120 with TMD, 25 with BMS, 19 with OL and 10 with TN. TMD patients were significantly younger than other groups (Table 2).
|
Characteristic |
TMD (n = 120) |
BMS (n = 25) |
OL (n = 19) |
TN (n = 10) |
Total (n = 174) |
|---|---|---|---|---|---|
|
Note: BMS = burning mouth syndrome, SD = standard deviation, TMD = temporomandibular disorder, TN = trigeminal neuralgia. OL = oral lesions/oral mucosal inflammation. |
|||||
| Age, years ± SD | 36.9 ± 17.2* | 57.32 ± 11.7 | 56.42 ± 14.7 | 67.5 ± 7.7 | 43.7 ± 18.9* |
| Female | 91 | 19 | 15 | 6 | Χ2 = 701† |
| Male | 29 | 6 | 4 | 4 | |
Overall, we found a significant difference between patient groups in 12 of the 17 items on the MFIQ; however, only 8 items showed significance on pairwise comparison. These items were taking a large bite, chewing hard foods, chewing resistant foods, yawning, eating meat, carrot, peanuts/almonds, and an apple. The sum, FIRS and MFIQ scores also showed a level of significance (Table 3, Figure 1).
|
MFIQ item |
Average rating ± SD |
p |
|||
|---|---|---|---|---|---|
|
TMD |
BMS |
OL |
TN |
||
|
*Indicates significance using the Kruskal-Wallis test. Bold type indicates significance between groups on the Dunn-Bonferroni post-hoc test. |
|||||
| Social activities | 0.55 ± 0.87 | 0.58 ± 1.18 | 0.37 ± 0.96 | 0.86 ± 0.90 | 0.262 |
| Speaking | 0.66 ± 1.0 | 0.40 ± 0.87 | 0.32 ± 0.67 | 0.44 ± 0.73 | 0.279 |
| Taking a large bite | 2.04 ± 1.29 | 0.56 ± 1.08 | 0.42 ± 0.96 | 1.89 ± 1.54 | < 0.001* |
| Chewing hard food | 1.98 ± 1.32 | 1.16 ± 1.57 | 1.00 ± 1.37 | 2.33 ± 1.50 | 0.002* |
| Chewing soft food | 0.67 ± 0.85 | 0.56 ± 1.23 | 0.26 ± 0.81 | 0.89 ± 1.17 | 0.028* |
| Work and/or daily activities | 0.78 ± 1.03 | 0.72 ± 1.17 | 0.16 ± 0.38 | 0.78 ± 0.97 | 0.059 |
| Drinking | 0.25 ± 0.55 | 0.56 ± 1.08 | 0.21 ± 0.54 | 0 | 0.336 |
| Laughing | 0.65 ± 0.88 | 0.48 ± 1.05 | 0.21 ± 0.71 | 0.30 ± 0.48 | 0.024* |
| Chewing resistant food | 1.83 ± 1.34 | 0.92 ± 1.55 | 0.74 ± 1.20 | 1.70 ± 1.34 | < 0.001* |
| Yawning | 1.57 ± 1.18 | 0.48 ± 1.05 | 0.42 ± 0.96 | 0.90 ± 1.66 | < 0.001* |
| Kissing | 0.71 ± 1.01 | 0.33 ± 0.87 | 0.37 ± 1.12 | 0.30 ± 0.95 | 0.025* |
| Eating | |||||
| A hard cookie | 1.27 ± 1.22 | 0.96 ± 1.43 | 0.95 ± 1.22 | 1.44 ± 0.882 | 0.172 |
| Meat | 1.62 ± 1.22 | 0.75 ± 1.36 | 0.84 ± 1.17 | 2.13 ± 1.46 | < 0.001* |
| A raw carrot | 1.69 ± 1.39 | 0.79 ± 1.41 | 1.05 ± 1.35 | 2.30 ± 1.25 | 0.001* |
| French bread | 1.51 ± 1.34 | 1.04 ± 1.55 | 0.83 ± 1.43 | 1.80 ± 1.55 | 0.032* |
| Peanuts/almonds | 1.21 ± 1.24 | 0.96 ± 1.51 | 0.49 ± 1.23 | 2.33 ± 1.32 | 0.013* |
| An apple | 1.57 ± 1.31 | 0.80 ± 1.50 | 0.89 ± 1.29 | 2.20 ± 1.48 | 0.001* |
| Sum | 20.17 ± 14.13 | 11.88 ± 17.42 | 9.79 ± 15.03 | 20.90 ± 14.33 | < 0.001* |
| FIRS | 1.93 ± 1.53 | 1.12 ± 1.62 | 0.79 ± 1.55 | 2.10 ± 1.5 | < 0.001* |
| MFIQ | 1.58 ± 0.67 | 1.28 ± 0.68 | 1.21 ± 0.63 | 1.60 ± 0.70 | 0.008* |
Pairwise comparison showed no significant difference between TMD and TN patients. Similarly, pairwise comparison showed no significant difference between BMS and OL patients.
TMD patients had significantly more difficulty than BMS and OL patients in taking a large bite (p < 0.001, p < 0.001), chewing hard foods (p = 0.036, p = 0.026), chewing resistant food (p = 0.004, p = 0.005), yawning (p < 0.001, p < 0.001) and eating meat (p = 0.002, p = 0.043) and more difficulty than BMS patients in eating raw carrots (p = 0.006) and apples (p = 0.010) (Figure 1a).
Sum and FIRS were significantly higher in TMD than BMS patients (p = 0.004, p = 0.035) and OL patients (p = 0.002, p = 0.002), and MFIQ score was significantly higher for TMD patients compared with OL patients (0.047).
Those with TN had significantly greater difficulty than OL patients in taking a large bite (p = 0.040) and eating peanuts/almonds (p = 0.019) and significantly greater difficulty than BMS patients in eating meat (p = 0.033), carrots (p = 0.009), peanuts/almonds (p = 0.024) and an apple (p = 0.021) (Figure 1b); they had a significantly higher FIRS than OL patients (p = 0.048).
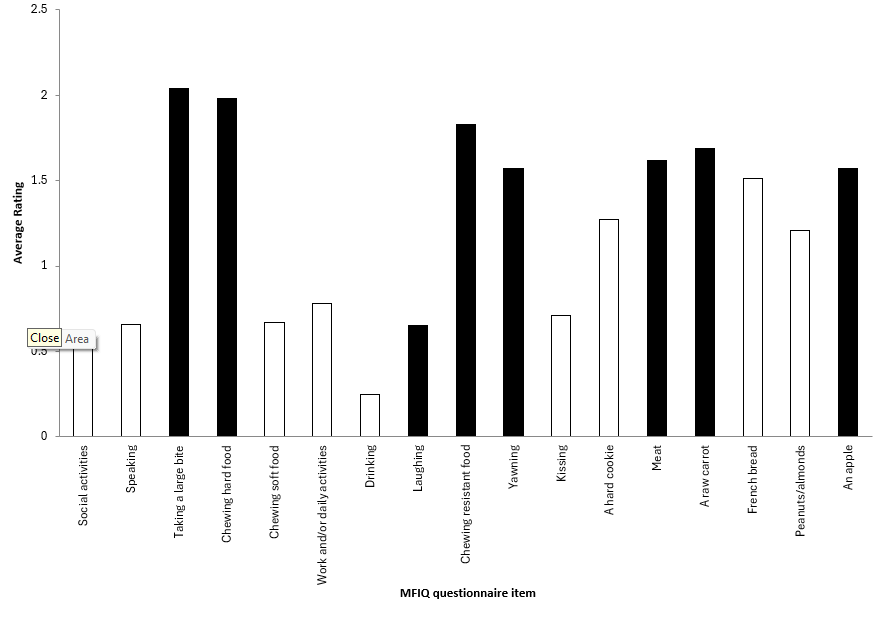
Figure 1a: Black bars indicate items rated significantly more difficult on the Mandibular Function Impairment Questionnaire (MFIQ) by patients with temporomandibular disorder (TMD) compared with patients with oral lesions/oral mucosal inflammation (OL) and/or burning mouth syndrome (BMS).
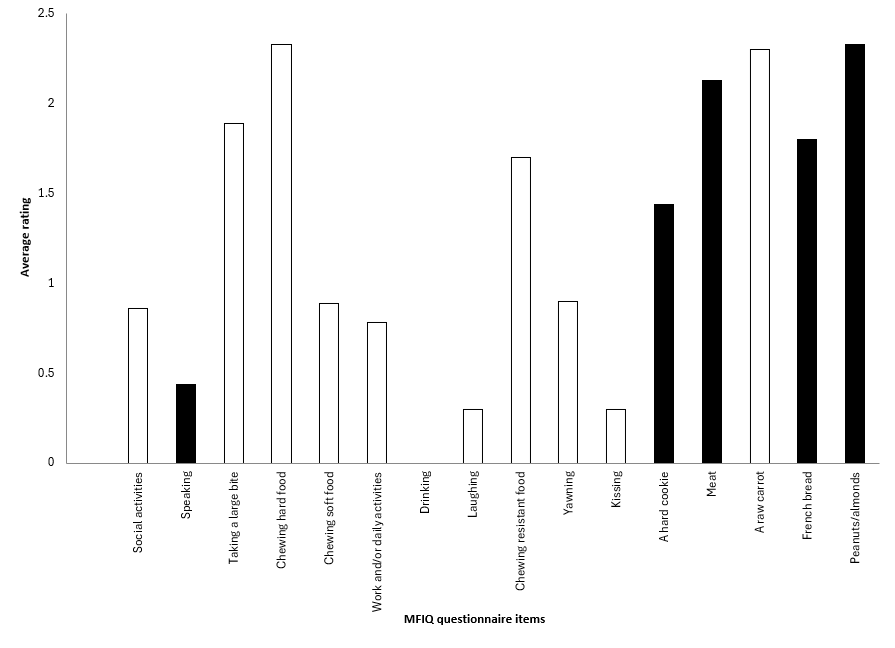
Figure 1b: Black bars indicate items rated significantly more difficult on the Mandibular Function Impairment Questionnaire (MFIQ) by patients with trigeminal neuralgia (TN) compared with patients with oral lesions/oral mucosal inflammation (OL) or burning mouth syndrome (BMS).
Discussion
Several items on the MFIQ questionnaire were ranked significantly more difficult by TMD patients. These included chewing hard and resistant foods (meat, raw carrots and apples) and functions associated with wide mouth opening (taking a large bite and yawning). TN patients had more difficulties with most food items, which may be related to sensitivity to touch associated with TN or to increased shooting pain with any jaw movement. TN is a neuralgic condition that most commonly affects the mandibular branch followed by the maxillary branch and then the ophthalmic branch of the trigeminal nerve, and it can be triggered by jaw movement.16 In 1 study, approximately 12% of patients with TMD had TN, which may explain why TN patients were not significantly different from TMD patients in their rankings on the MFIQ.17
BMS is prevalent in post-menopausal women.18 Approximately 66% of BMS patients show signs and symptoms of TMD, and parafunctional habits have been reported in 20–73% of BMS patients.19 However, our study did not show a high global score on the MFIQ for this population, likely because the TM joints and associated musculature were not inflamed, even though degenerative changes are more commonly found in older patients.20 As Niezen21 et al. pointed out, because the MFIQ functions as a subjective pain scale, it is likely our BMS population did not perceive pain associated with their jaw, as their oral symptoms were a more pressing concern and may have masked any jaw-related issues. Similarly, OL patients who may have clinical signs of TMD, including joint noises and/or deviation on mouth opening without complaint of jaw pain, did not score highly on the MFIQ, likely because of a lack of jaw pain.
Both TMD and TN patients had high MFIQ global scores. This is consistent with Niezen21 et al.’s observation that a higher score on the MFIQ is often associated with the presence of pain, suggesting that the MFIQ global score functions as a pain indicator.
Although the MFIQ global score was significantly higher in TMD patients compared with those with primarily oral complaints, it was not effective in differentiating facial pain originating from TMD and TN. This study included only a small number of TN patients, and the results may differ for a larger population. In addition, our TN patients were also referred for jaw assessment; thus, they may not be representative of all patients with this condition. A study involving a larger population of TN patients would better assess the application of the MFIQ in differentiating TMD from TN.
Overall, this study suggests that the MFIQ global score is not effective in differentiating pain caused by TMD from referred orofacial pain. However, a high rating of difficulty in taking a large bite, chewing hard food, chewing resistant food and yawning, together with a complaint of facial pain may be helpful in guiding general practitioners to consider TMD and possibly TN examination of these patients in addition to assessing them for dental cause of pain.
Conclusion
The MFIQ global score is not effective in the clinical diagnosis of TMD. However, if patients indicate great difficulty in taking a large bite, chewing hard foods, chewing resistance food and yawning, clinical assessment should take into consideration the possibility of TN and TMD as the cause of orofacial pain.
THE AUTHORS
Corresponding author: Dr. Miriam Grushka, 974 Eglinton Ave. W, Toronto ON M6C 2C5; miriamgrushka@gmail.com.
The authors have no declared financial interests.
This article has been peer reviewed.
References
- Pigg M, Nixdorf DR, Law AS, Renton T, Sharav Y, Baad-Hansen L, et al. New International Classification of Orofacial Pain: what is in it for endodontists? J Endod. 2021;47(3):345-57.
- Ananthan S, Benoliel R. Chronic orofacial pain. J Neural Transm (Vienna). 2020;127(4):575-88.
- Slade GD, Ohrbach R, Greenspan JD, Fillingim RB, Bair E, Sanders AE, et al. Painful temporomandibular disorder: decade of discovery from OPPERA studies. J Dent Res. 2016 Sep;95(10):1084-92.
- Stegenga B, de Bont LG, de Leeuw R, Boering G. Assessment of mandibular function impairment associated with temporomandibular joint osteoarthrosis and internal derangement. J Orofac Pain. 1993;7(2):183-95.
- Chen YY, Fan HC, Tung MC, Chang YK. The association between Parkinson's disease and temporomandibular disorder. PLoS One. 2019;14(6):e0217763.
- Nam Y, Kim HG, Kho HS. Differential diagnosis of jaw pain using informatics technology. J Oral Rehabil. 2018;45(8):581-8.
- Gharavi SM, Qiao Y, Faghihimehr A, Vossen J. Imaging of the temporomandibular joint. Diagnostics (Basel). 2022;12(4):1006.
- Differential diagnosis and management of TMDs. In: Klasser GD, Romero Reyes M, editors. Orofacial pain: guidelines for assessment, diagnosis, and management (7th ed.). Batavia, Ill.: Quintessence Publishing; 2023: p. 189-261.
- Bendtsen L, Zakrzewska JM, Heinskou TB, Hodaie M, Leal PRL, Nurmikko T, et al. Advances in diagnosis, classification, pathophysiology, and management of trigeminal neuralgia. Lancet Neurol. 2020;19(9):784-96.
- Neuropathic pain. In: Klasser GD, Romero Reyes M, editors. Orofacial pain: guidelines for assessment, diagnosis, and management (7th ed.). Batavia, Ill.: Quintessence Publishing; 2023: p. 121-62.
- Fitzpatrick SG, Cohen DM, Clark AN. Ulcerated lesions of the oral mucosa: clinical and histologic review. Head Neck Pathol. 2019;13(1):91-102.
- Oral mucosa pain. In: Klasser GD, Romero Reyes M, editors. Orofacial pain: guidelines for assessment, diagnosis, and management (7th ed.). Batavia, Ill.: Quintessence Publishing; 2023: p. 172-82.
- Klasser GD, Grushka M, Su N. Burning mouth syndrome. Oral Maxillofac Surg Clin North Am. 2016;28(3):381-96.
- Su N, Poon R, Liu C, Dewan C, Darling M, Grushka M. Taste and pain response in burning mouth syndrome with and without geographic tongue. J Oral Facial Pain Headache. 2020;34(3):217-21.
- Poon R, Su N, Ching V, Darling M, Grushka M. Reduction in unstimulated salivary flow rate in burning mouth syndrome. Br Dent J. 2014;217(7):E14.
- Gerwin R. Chronic facial pain: trigeminal neuralgia, persistent idiopathic facial pain, and myofascial pain syndrome: an evidence-based narrative review and etiological hypothesis. Int J Environ Res Public Health. 2020;17(19):7012.
- Dupont Jr JS. The prevalence of trigeminal neuritis with TMD. Cranio. 2003;21(3):180-4.
- Russo M, Crafa P, Guglielmetti S, Franzoni L, Fiore W, Di Mario F. Burning mouth syndrome etiology: a narrative review. J Gastrointestin Liver Dis. 2022;31(2):223-8.
- Corsalini M, Di Venere D, Pettini F, Lauritano D, Petruzzi M. Temporomandibular disorders in burning mouth syndrome patients: an observational study. Int J Med Sci. 2013;10(12):1784-9.
- Su N, Poon R, Friedman L, Darling M, Grushka M. TMJ changes in adolescent TMD patients seen on MRI in clinical setting. N Y State Dent J. 2015;81(3):27-30.
- Niezen ET, Bos RRM, de Bont LGM, Stegenga B, Dijkstra PU. Complaints related to mandibular function impairment after closed treatment of fractures of the mandibular condyle. Int J Oral Maxillofac Surg. 2010;39(7):660-5.



