Abstract
Infective endocarditis (IE) remains one of the most serious diseases with a high morbidity and mortality rate. Although the condition is more common in the medical field in a hospital setting, dentists must have a thorough understanding of the overall pathogenesis, epidemiology, risk factors and signs and symptoms that may be present in their patient population. In 2021, the American Heart Association (AHA) updated its guidelines on IE, emphasizing the specific criteria that put a patient at risk of acquiring IE, specific dental procedures that can increase the risk of IE by inducing bacteremia and an antibiotic prophylaxis regimen to act as a preventive measure if needed. This literature review gives the dental practitioner a general overview of the AHA guidelines as well as information on prevention in their at-risk patients and the need to emphasize a well-structured, consistent daily oral hygiene routine.
Infective endocarditis (IE) is an infection of the endocardium, the innermost lining of the heart.1 It arises when bacteria from the bloodstream attach to damaged or roughened endothelial surfaces of the heart. IE occurs in those with normal or damaged heart valves, although it tends to be more common in the latter. The American Heart Association (AHA) recently updated its guidelines for IE in 20211 consolidating its guidelines from 20072, with antibiotic prophylaxis (AP) no longer recommended for over 90% of patients who were previously included, and a shift in focus toward oral hygiene as opposed to reliance on antibiotics. In 2023, the European Society of Cardiology (ESC) also updated its guidelines3 for IE aligning with those of the AHA 2021 guidelines.1 The importance of these updates to the dental practitioner is to reinforce their knowledge of the etiology, epidemiology and overall nature of IE to allow them to provide more comprehensive care to their patients who are at increased risk of this preventable disease, which is associated with both high morbidity and high mortality.
Methods
This review involved a detailed search of peer-reviewed journal articles using the University of Toronto library system. Most guidelines originate from advisory bodies in the United States (AHA) and Europe (ESC). Electronic databases accessed include PubMed, MEDLINE, ScienceDirect and the Cochrane Library. In all, 468 articles were identified, 74 were investigated and 36 are cited in this review. Some of the major keywords used in the search were “infective endocarditis,” “infective endocarditis guidelines,” “dental procedures that trigger infective endocarditis” and “infective endocarditis epidemiology.” Over 70% of the content reported here is from scientific reviews and peer-reviewed manuscripts published in the last 7 years. Table 1 shows the inclusion and exclusion criteria.
|
Inclusion criteria |
Exclusion criteria |
|---|---|
|
|
Epidemiology
IE affects 15 in 100 000 individuals in the United States, with its incidence increasing annually. 4 The ESC’s 2019 estimated global incidence is similar at 13.8 cases per 100 000 and 0.87 deaths per 100 000 population annually. 3 These numbers are increasing: a 17-year study from Italy5 also demonstrated an increasing annual incidence of IE of 4.6/100 000 population; the dominant reasons for increasing incidence and mortality were cited as an aging population and more cases of staphylococcus and other infections associated with invasive procedures. The prognosis for IE tends to be poor, with a mortality rate of ~30% after 30 days of disease progression.6 Furthermore, health care-associated IE accounts for 25–30% of cases because of greater use of IV lines and intracardiac devices. 6 IE tends to be more common in men than women, and its incidence increases with age. 7 In addition to the physical consequences of IE, psychological affects often go unnoticed and are rarely discussed in the literature. People who survive IE demonstrate a decreased quality of life and evidence of posttraumatic stress disorder. 8
Methods
Review of Pathologically Relevant Heart Anatomy The heart is located in the middle mediastinum. It is surrounded by the pericardium, which is composed of 2 layers: fibrous (a visceral layer also known as the epicardium) and serous (the parietal layer). 9 The heart contains 2 types of valves: atrio-ventricular (AV) valves — the tricuspid valve on the right side of the heart and the bicuspid/mitral valve on the left; and semilunar valves — the pulmonary valve on the right and the aortic on the left (Figure 1). 9
The AV valves are pulled by the chordae tendineae, which connect to the papillary muscles responsible for contraction along with the ventricles which help seal the valve cusps. 9 During diastole, the relaxed part of the heartbeat, the AV valves are open (papillary muscles are relaxed) and the ventricles fill with blood from the atria. Systole is the contraction stage, in which the AV valves are shut (because of pressure and tension in the papillary muscles).9 The heart anatomy can be reviewed in Figure 1.10
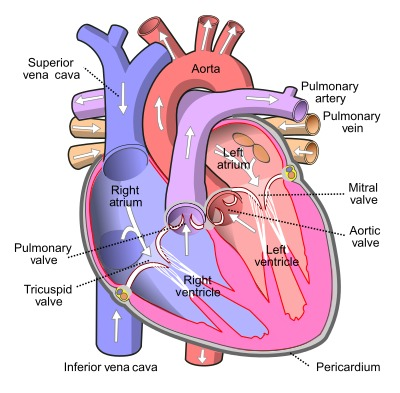
Figure 1: Heart anatomy. Source: Pierce 2024.10
Predisposing/Risk Factors
IE is associated with many risk factors of which a health care practitioner must be aware, both cardiac and non-cardiac. Cardiac risk factors include: prosthetic valves, congenital heart defects, rheumatic heart disease, mitral valve prolapse, aortic valve stenosis and the presence of an implantable electronic cardiac device.11 Hypertrophic cardiomyopathy paired with subaortic stenosis or ventricular aneurysm is also a risk factor.12 Non-cardiac risk factors include: diabetes mellitus, hemodialysis, IV drug use, immunosuppression and poor oral hygiene.11 The contribution of these risk factors is further explored below (see Pathogenesis). Knowledge of these risk factors is imperative, as they contribute to the diagnosis of IE, which should be considered in all patients presenting with sepsis or a fever of unknown origin.3
Pathogenesis
IE occurs along the endothelial layer of the heart, including the heart valves, which compartmentalize the heart chambers and control blood flow between them in an organized manner (Figure 2). For IE to occur and progress, bacteria must first enter the bloodstream (bacteremia). When a heart valve is damaged or surgically operated on (typically through prosthetic valves), the bacteria adhere to and colonize it. Although IE is initiated by the presence of bacteremia, it is important to consider host factors that are also involved in the pathogenesis of IE, i.e., leukocytes, platelets and the valve surfaces.13
Left-side IE, seen on the aortic and mitral valves, is more common than right-side IE, which is found on the tricuspid valve.14 Progressive growth of bacteria forms vegetations, which consist of a combination of fibrin, platelets and antibodies produced by the host’s immune system to contain the infection.15 Bacteria involved in the propagation of the initial adherence include Streptococcus viridans, the most common cause of IE in both damaged and abnormal valves, and Staphylococcus aureus, which is found in both normal and damaged valves, most commonly in IV drug users.15 When the tricuspid valve is affected, the most common pathogen is Staphylococcus aureus.15
Acute forms of IE are usually caused by the highly virulent Staphylococcus aureus, with mortality higher than the subacute form and characterized by larger vegetations.6 The acute form has been found to start more abruptly and progress more rapidly. This virulent bacterium was concluded to be the causative organism in a third of patients diagnosed with IE globally.16 Subacute forms involve the less virulent Streptococcus viridans and are characterized by smaller vegetations on damaged or diseased heart valves.6 Compared with the acute form, the subacute form starts more subtly, its progression is slower over time and it most commonly affects the mitral and aortic valves (Table 2).17
|
Bacteria |
Form of IE |
Vegetation size |
Valves affected |
Consequence |
|---|---|---|---|---|
| Staphylococcus aureus | Acute | Relatively large |
|
Acute and rapid progression of IE |
| Streptococcus viridans | Subacute | text |
|
IE arises subtly and progresses more slowly |
| *Organisms in the HACEK group— Haemophilus parainfluenzae, H. aphrophilus, Aggregatibacter actinomycetemcomitans, Cardiobacterium hominis and Eikenella and Kingella species — have also been found to contribute to the pathogenesis and progression of IE, but are less significant because of their far lower incidence and prevalence.12 | ||||
Clinical Presentations, Symptoms and Diagnosis
The clinical presentation and symptoms of IE vary depending on the causative pathogen. Constitutional symptoms, including fever, weight loss and night sweats, are highly indicative of IE.12 Dermatologic lesions, which include Osler nodes, Janeway lesions and petechiae, are uncommon but highly suggestive of IE. Osler nodes are hemorrhagic lesions that tend to localize in the fingers, hands or feet, making them tender on palpation and, therefore, painful.18 Janeway lesions are similar hemorrhagic lesions, but are macular papules localized on the soles of the feet or palms of the hand; they are painless and are the result of microemboli.18
The dental practitioner must pay attention to Roth spots and certain cardiac symptoms as indicators of a patient presenting with IE. Roth spots are white-centred hemorrhages localized in the retina.18 Once again, although relatively uncommon, they are serious clinical presentations and highly indicative of IE. Cardiac symptoms of clinical importance include congestive heart failure,7 heart block, valve rupture and regurgitation.18 Heart failure was found to be the most common cause of death for a patient diagnosed with IE.19
Although clinical presentations and symptoms are important, one must also be aware of the many diagnostic tools available to aid in reaching a confident diagnosis of IE and ruling out other conditions. One such tool that is used extensively is modified Duke criteria.20 The criteria are divided into 2 categories based on severity: major and minor. Major criteria include positive blood cultures and endocardial involvement. Minor criteria include fever, predisposing heart condition or IV drug use, vascular phenomena, immunologic phenomena and microbiological evidence other than a positive blood culture.
Diagnosis may be definite, possible or rejected IE.20 Definite IE meets 2 major criteria, 1 major and 3 minor criteria or 5 minor criteria. Possible IE would include 1 major and 1 minor criterion or 3 minor criteria. Finally, rejected IE would be declared when the health care professional has made an alternative diagnosis, has found no pathological evidence of IE at surgery or the condition does not meet any of the criteria listed above.20
Nonetheless, IE may be challenging to diagnose, as its presentation could be acute with fever or subacute with low-grade to no fever; non-specific symptoms could mimic other diseases and misdirect the clinician. 3 Suspicion of IE usually arises from fever and positive blood cultures when an alternative diagnosis of infection is unlikely.3 The clinician will usually rely on cardiac and non-cardiac risk factors along with the modified Duke criteria; however, to make matters more complex, the overall sensitivity of the modified Duke criteria is 80%,21 because of IE’s highly variable clinical presentations. Thus, the ESC recommends a multimodal imaging approach with echocardiography as the gold standard, but also including other modalities, such as computed tomography and nuclear imaging. 22-24
Discussion
Relevance of IE to the Dental Practitioner
Patients at Risk of IE — The 2021 revision of the AHA guidelines maintain the grouping of high-risk viridans group streptococcal IE susceptible individuals into the same 4 groups they established in 2007 (Table 3). Both the European and American guidelines do not recommend AP, except for high-risk cases, 1,3 consequently excluding patients with mitral valve prolapse, rheumatic heart disease and other conditions that are not listed in Table 3, from receiving AP for infective endocarditis. However, such patients could undergo mitral valve repair, preferably through a minimally invasive procedure or possibly open-heart surgery, which would warrant the use of AP according to the AHA. 1 It is important to note that patients could require procedures that would elevate their risk of IE and, therefore, warrant the use of AP. Thus, it is imperative for the dental practitioner to keep the medical history of their patients up to date. Moreover, the AHA recommendations are only guidelines, and it is up to the dental practitioner to decide whether a patient would benefit from the use of AP. 1
|
Source: Habib et al. 2019.19 *A 10-year retrospective hospital study34 of adult patients diagnosed using the Duke criteria found mortality of prosthetic valve IE to be 26.8% compared with 16.5% for native valve IE. †Risk of heart failure, need for cardiac valve replacement surgery and mortality rates are higher for patients with recurrent IE than for patients with their first exposure to a native valve IE episode, especially for recurrent IE intravenous drug use patients.35 Moreover, patients who experience multiple episodes of IE, whether native or prosthetic valve, are at greater risk of further episodes of IE with severity increasing with each episode.36 ‡Children with CHD are at most risk of IE in middle- and high-income countries, especially those with complex cyanotic heart disease and those who have postoperative palliative shunts, conduits or other prostheses.37 According to the Pediatric Health Information System Database, 68% of patients admitted with IE between 2003 and 2010 had CHD.38 §Although there is insufficient evidence showing the risk of adverse outcomes from IE in cardiac transplant recipients who develop valvulopathy, these patients are immunosuppressed and have various underlying comorbidities, making them highly susceptible to IE adverse outcomes, and hence antibiotic prophylaxis is warranted.1 |
1. Prosthetic cardiac valve or material used for cardiac valve repair*
|
| 2. Previous, relapse or recurrent IE† |
3. The following congenital heart disease (CHD) conditions‡
|
| 4. Cardiac transplant recipients who develop valvulopathy§ |
Some notable conditions that do not require AP include congestive heart failure, rheumatic heart disease, coronary artery stents, septal defect closure devices when complete closure is attained, peripheral vascular grafts and patches, including the ones used for hemodialysis, central nervous system ventriculoatrial shunts, vena cava filters, certain implantable electronic devices (i.e. pacemaker), pledgets, completely repaired CHD defect with prosthetic material 6 months or more after the procedure and cardiac transplant patients who do not develop valvulopathy (Table 4). 1
|
AP indicated |
AP not indicated |
|---|---|
|
Source: Wilson et al. 2021.1 |
|
| Suture removal | Local anesthetic injection through non-infected tissue |
| Biopsy | Capturing dental radiographs |
| Placing orthodontic bands | Placing orthodontic brackets |
| Extractions | Shedding primary teeth |
| Periodontal probing | Insertion of removable prosthodontic appliances |
| Subgingival restorations | Adjustment of orthodontic appliances/braces or placing removable orthodontic appliances |
| Periodontal surgery | Bleeding of lips or oral mucosa resulting from trauma |
Dental procedures that can induce IE in the 4 susceptible groups — For patients in the 4 high-risk groups (Table 3), the dental practitioner must be aware that the dental procedures outlined in Table 4 can increase the risk of infective endocarditis and, hence, AP is suggested. This includes all dental procedures that involve the manipulation of the gingiva or the periapical region of the teeth or perforation of the oral mucosa.25
Meanwhile, AP is not suggested for injection of anesthetic through non-infected tissue, taking dental radiographs, placing removable prosthodontic or orthodontic appliances and brackets, exfoliation of primary teeth and trauma-induced bleeding of the lips or oral mucosa.1
Prevention of IE using antibiotic prophylaxis — Now that the dental practitioner understands the risk of IE associated with certain dental procedures for specific populations, the best prevention requires administering AP according to protocol (Table 5) in a single dose 30–60 minutes before the dental procedure.1 The benefits of administering AP significantly outweigh the risk of anaphylactic shock, hives, angioedema or other significant immunological phenomena, which is low in this context.1
|
Type of administration |
AP agent |
|---|---|
|
*Administered as a single dose 30–60 minutes before an indicated dental procedure as stipulated by the 2021 AHA guidelines1 and the 2023 ESC guidelines. 3 |
|
| Oral | Amoxicillin
|
| Intramuscular (IM) or intravascular (IV) (unable to take oral medication) | Ampicillin
|
Cefazolin or ceftriaxone†‡
|
|
| Oral (penicillin or ampicillin allergy) | Cephalexin
|
Azithromycin or clarithromycin§
|
|
Doxycycline
|
|
| IM or IV (penicillin or ampicillin allergy and unable to take oral medication) | Cefazolin or ceftriaxone
|
Although previously used, clindamycin is no longer recommended, as a single use of it could result in complications, including death, from Clostridioides difficile infection.26 Its prescription for dental procedures accounts for up to 15% of community-acquired C. difficile infections.27 Other classes of antibiotics carry minimal risk of side effects; cephalexin, cefazolin and ceftriaxone, which all belong to the class of cephalosporins and possess fewer significant side effects than many other classes of antibiotics, such as beta-lactams.28 Macrolides, a class of antibiotics that includes clarithromycin, are a much better alternative to clindamycin as they do not result in C. difficile infection, but they should be used with caution as they could result in serious cardiovascular events, such as torsades de pointes, ventricular tachycardia and ventricular fibrillation in patients known to have a prolonged QTc interval.29 Doxycycline is a good alternative for patients who have penicillin allergy and cannot tolerate cephalosporin or macrolide, as a severe reaction from a single dose of doxycycline is extremely rare.
The antibiotic coverage period is an important factor to consider when administering antibiotics. A single 2-g oral dose of amoxicillin should result in a minimal inhibitory concentration (MIC) for at least 6 h. This is because amoxicillin has a half-life of approximately 80 min, with the average peak plasma concentration of 4 μg/mL achieved after 2 h of oral administration.30 Because penicillin-sensitive S. viridans has an MIC of 0.2 μg/mL,30 a 2-g dose of amoxicillin would result in at least 6 h of acceptable MIC. Hence, if a dental procedure will take longer than 6 h, the dentist is encouraged to administer a second dose of the previously used antibiotic. If an antibiotic dose was not administered before a dental procedure, it would be prudent to administer it up to 2 h after the procedure.30 However, if an antibiotic is not administered within this time frame, no antibiotic is recommended, but the dentist should inform the patient of the situation and contact their family physician to discuss the matter with the patient as soon as possible.30
Antibiotic Resistance
It is imperative for the dentist to administer AP according to approved guidelines, as failure to do so could lead to antibiotic resistance. The Centers for Disease Control and Prevention (CDC) in the United States estimate that, in 2019, antibiotic-resistant infections were responsible for >2.8 million infections, 35 000 of which led to death and cost $10s of billions in health care expenses.31 Certain antibiotics, such as the macrolide clarithromycin, are more likely to contribute to the development of resistance than a beta-lactam, such as penicillin.1 Nonetheless, as mentioned previously, there was no evidence sufficient for the AHA to change its 2007 AP guidelines.1
A protocol to reduce the risk of antibiotic resistance in IE-susceptible patients, as established in AHA’s 2007 guidelines and retained in the 2021 guidelines, goes as follows: if a second appointment is necessary for the patient and they must return for a dental procedure that requires AP within 10 days of having an antibiotic administered, a different class of antibiotic is recommended.1 For example, using clarithromycin at the second appointment if amoxicillin was previously administered. This includes both antibiotics used for infections and prophylactic purposes, whether prescribed by the dentist or another physician. If the second appointment is elective, it is preferable to delay it for at least 10 days from the last administration of antibiotics.1 In the latter scenario, choosing an alternative class of antibiotic would not be necessary.
Clinical Considerations
The dental practitioner should now understand the overall nature of IE, the causative bacteria present, populations at risk of acquiring IE, ways in which they can help prevent IE and specific procedures that require AP. However, they should also know what previous concepts no longer apply, such as AP for rheumatic heart disease. Furthermore, the dental practitioner should also be confident in the medical realm that surrounds IE and educate their patients who are at risk of this disease.
Rheumatic heart disease is an inflammatory condition resulting from rheumatic fever. Rheumatic fever arises from group A beta-hemolytic streptococcal pharyngitis, also known as “strep throat.” The host produces antibodies against the streptococcal bacteria, which can damage cardiac valves as the antigens of both the A beta-hemolytic streptococcal bacteria and the antibodies are similar in structure. The result is fibrosis and damage to the valves, with the mitral valve being the most susceptible. Dentists should be aware that the administration of AP for IE in patients with pre-existing rheumatic heart disease is no longer required for this disease since publication of the 2007 AHA guidelines.2
In terms of prevention, patients with the underlying cardiac conditions described in Table 3, should be educated by their dental team on the importance of consistent and effective oral hygiene to mitigate the risk of acquiring IE. Patients should understand that reducing the oral bacterial load is the most effective way to prevent IE. Proper oral hygiene — tooth brushing, flossing and using antiseptic mouthwash — not only reduces the load but also allows the patient to be an active part of the dental team in preventing IE.32 According to a review article,33 good oral hygiene and the regular use of fluoride can help reduce the risk of an oral infection, along with regular dental cleaning and, when appropriate, AP. The AHA also concludes that, although they recommend the use of AP for all high-risk patients, dental practitioners must also impress upon all their patients the critical need for good oral hygiene and regular dental visits.1 When thorough and consistent oral hygiene is combined with AP among patients at risk of IE, the risk is significantly decreased; this should be used as an incentive to for patients to improve and perform their oral care routine daily.1
In summary, this review highlights the most important points regarding IE and the considerations surrounding it. The topic can seem intimidating, considering the knowledge practitioners must have to understand the signs and symptoms of IE, AP regimens and the specific dental procedures that warrant AP. We hope this review has done an effective job at summarizing key points for the dental practitioner with patients who are at risk of developing IE. Our review is based on research published in up-to-date peer-reviewed journals and incorporates the highlights of the most recent AHA guidelines.
A shortcoming of this review is the focus of the research articles on longitudinal studies, retrospective research and case studies. Ideally, randomized controlled trials and experimental methods should be included to further investigate the nature of IE and cause–effect relationships. Thus, we suggest that future literature reviews of IE incorporate experimental research to help answer questions general practitioners may have, fortify conclusions that can be made with tangible results and propel further research on IE and dentistry.
Conclusion
Dental practitioners must have a clear and informed understanding of IE as a whole. The AHA gives the dental community clear guidelines on what to look for in their patient population, specific dental procedures that may increase the risk of IE and recommendations for AP for patients who would benefit from such treatment. It is likely that the guidelines will change in the future because of ongoing advancements in the study of IE. However, regardless of whether patients are at risk of IE, the dental practitioner should always recommend consistent and effective oral hygiene, as being of utmost importance in advancing oral health and reducing risk of infectious disease.
THE AUTHORS
Corresponding author: Dr. Aviv Ouanounou, Dept. of Clinical Sciences, Pharmacology and Preventive Dentistry, Faculty of Dentistry, University of Toronto, 124 Edward St., Room 370, Toronto ON M5G 1G6. Email: aviv.ouanounou@dentistry.utoronto.ca
Note from Corresponding Author: “Both co-authors contributed equally to this manuscript.”
The authors have no declared financial interests.
This article has been peer reviewed.
References
- Wilson WR, Gewitz M, Lockhart PB, Bolger AF, DeSimone DC, Kazi DS, et al. Prevention of viridans group streptococcal infective endocarditis: a scientific statement from the American Heart Association. Circulation. 2021;143:e963-78.
- Wilson W, Taubert KA, Gewitz M, Lockhart, PB, Baddour LM, Levison M, et al. Prevention of infective endocarditis: guidelines from the American Heart Association: a guideline from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation. 2007;116(15):1736-54.
- Delgado V, Marsan NA, de Waha S, Bonaros N, Brida M, Burri H, et al. 2023 ESC guidelines for the management of endocarditis. European Heart Journal. 2023;44(39):3948-4042.
- Pant S, Patel NJ, Deshmukh A, et al. Trends in infective endocarditis incidence, microbiology, and valve replacement in the United States from 2000 to 2011. Journal of the American College of Cardiology. 2015;65(19):2070-2076.
- Cresti A, Chiaverelli M, Scalese M, Nencioni C, Valentini S, Guerrini F, et al. Epidemiological and mortality trends in infective endocarditis, a 17-year population-based prospective study. Cardiovasc Diagn Ther. 2017;7(1):27-35.
- Rajani R, Klein JL. Infective endocarditis: a contemporary update. Clin Med (Lond). 2020;20(1):31-5.
- Ambrosioni J, Hernandez-Meneses M, Tellez A, Pericàs J, Falces C, Tolosana JM, et al. The changing epidemiology of infective endocarditis in the twenty-first century. Curr Infect Dis Rep. 2017;19(5):21.
- Bagnasco MS, Nuñez-Gil IJ. Infective endocarditis and thoracic aortic disease: a review on forgotten psychological aspects. World J Cardiol. 2017;9(7):620-8.
- Anderson RH, Razavi R, Taylor AM. Cardiac anatomy revisited. J Anat. 2004;205(3):159-177.
- Pierce E. Blood flow through the heart. In: Bamalan OA, Jozsa F, Soos MP (editors). Anatomy, thorax, heart great vessels. Treasure Island, Fla.: StatPearls Publishing; 2024. Available: https://www.ncbi.nlm.nih.gov/books/NBK547680/figure/article-22425.image.f8/ (accessed 2024 Apr. 25).
- Cahill TJ, Dayer M, Prendergast B, Thornhill M. Do patients at risk of infective endocarditis need antibiotics before dental procedures? BMJ. 2017;358:j3942.
- Ashley EA, Niebauer J. Chapter 10: infective endocarditis. In: Cardiology explained. London: Remedica; 2004. Available: https://www.ncbi.nlm.nih.gov/books/NBK2208/ (accessed 2022 Aug. 10).
- Sullam PM, Drake TA, Sande MA. Pathogenesis of endocarditis. Am J Med. 1985;78(6B):110-5.
- Hussain ST, Witten J, Shrestha NK, Blackstone EH, Pettersson GB. Tricuspid valve endocarditis. Ann Cardiothorac Surg. 2017;6(3):255-61.
- Di Mauro M, Foschi M, Dato GMA, Centofanti P, Barili F, Corte AD, et al. Surgical treatment of isolated tricuspid valve infective endocarditis: 25-year results from a multicenter registry. Int J Cardiol. 2019;292:62-7.
- Cahill TJ, Baddour LM, Habib G, Hoen B, Salaun E, Pettersson GB, et al. Challenges in infective endocarditis. J Am Coll Cardiol. 2017;69(3):325-44.
- Wu Z, Chen Y, Xiao T, Niu T, Shi Q, Xiao Y. Epidemiology and risk factors of infective endocarditis in a tertiary hospital in China from 2007 to 2016. BMC Inf Dis. 2020;20(1):428.
- Long B, Koyfamn A. Infectious endocarditis: an update for emergency clinicians. Am J Emerg Med. 2018;36(9):1686-92.
- Habib G, Erba PA, Iung B, Donal E, Cosyns B, Laroche C, et al. Clinical presentation, aetiology and outcome of infective endocarditis. Results of the ESC-EORP EURO-ENDO (European infective endocarditis) registry: a prospective cohort study. Eur Heart J. 2019;40(39):3222-32.
- Habib G, Lancellotti P, Antunes MJ, Bongiorni MG, Casalta JP, Del Zotti F, et al. 2015 ESC guidelines for the management of infective endocarditis: the Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J. 2015;36(44):3075-128.
- Li JS, Sexton DJ, Mick N, Nettles R, Fowler Jr VG, Ryan T, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. 2000;30(4): 633-8.
- Kim I-C, Chang S, Hong G-R, Lee SH, Lee S, Ha J-W, et al. Comparison of cardiac computed tomography with transesophageal echocardiography for identifying vegetation and intracardiac complications in patients with infective endocarditis in the era of 3-dimensional images. Circulation: Cardiovascular Imaging. 2018;11:e006986.
- Salaun E, Habib G. Beyond standard echocardiography in infective endocarditis: computed tomography, 3-dimensional imaging, and multi-imaging Circulation: Cardiovascular Imaging. 2018;11:e007626.
- Pizzi MN, Roque A, Fernandez-Hidalgo N, Cuellar-Calabria H, Ferreira-Gonzalez I, Gonzalez-Alujas MT, et al. Improving the diagnosis of infective endocarditis in prosthetic valves and intracardiac devices with 18F-fluordeoxyglucose positron emission tomography/computed tomography angiography: initial results at an infective endocarditis referral center. Circulation. 2015;132:1113–1126.
- Tubiana S, Blotière PO, Hoen B, Lesclous P, Millot S, Rudant J, et al. Dental procedures, antibiotic prophylaxis, and endocarditis among people with prosthetic heart valves: nationwide population based cohort and a case crossover study. BMJ. 2017;358:j3776.
- Thornhill MH, Dayer MJ, Prendergast B, Baddour LM, Jones S, Lockhart PB. Incidence and nature of adverse reactions to antibiotics used as endocarditis prophylaxis. J Antimicrob Chemother. 2015;70(8):2382-8.
- Thornhill MH, Dayer M, Lockhart PB, Prendergast B. Antibiotic prophylaxis of infective endocarditis. Curr Infect Dis Rep. 2017;19(2):9.
- Macy E. Why was there ever a warning not to use cephalosporins in the setting of a penicillin “Allergy”? J Allergy Clin Immunol Pract. 2021;9(11):3929-33.
- Patel PH, Hashmi MF. Macrolides. Treasure Island, Fla.: StatPearls Publishing; 2024. Available: https://www.ncbi.nlm.nih.gov/books/NBK551495/ (accessed 2024 Apr. 25).
- Little JW, Miller CS, Rhodus NL. Chapter 2: infective endocarditis. In: Little and Falace’s dental management of the medically compromised patient (9th ed.). St. Louis, Mo.: Elsevier; 2023. p. 19-37.
- Antibiotic resistance threats in the United States 2019. Atlanta: Centers for Disease Control and Prevention; 2019. Available: https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf (accessed 2024 Apr. 25).
- Del Giudice C, Vaia E, Liccardo D, Marzano F, Valletta A, Spagnuolo G, et al. Infective endocarditis: a focus on oral microbiota. Microorganisms. 2021;9(6):1218.
- Coll PP, Lindsay A, Meng J, Gopalakrishna A, Raghavendra S, Bysani P, et al. The prevention of infections in older adults: oral health. J Am Geriatr Soc. 2019;68(2):411-6.
- Angsutararux T, Angkasekwinai N. Cumulative incidence and mortality of infective endocarditis in Siriraj hospital–Thailand: a 10-year retrospective study. BMC Infect Dis. 2019;19(1):1062.
- Citro R, Chan KL, Miglioranza MH, Laroche C, Benvenga RM, Furnaz S, et al. Clinical profile and outcome of recurrent infective endocarditis. Heart. 2022;108(21):1729-37.
- Pallasch TJ, Wahl MJ. Focal infection: new age or ancient history? Endod Topics. 2003;4(1):32-45. Available: https://onlinelibrary.wiley.com/doi/abs/10.1034/j.1601-1546.2003.00002.x (accessed 2024 Apr. 25).
- Day MD, Gauvreau K, Shulman S, Newburger JW. Characteristics of children hospitalized with infective endocarditis. Circulation. 2009;119(6):865-70.
- Pasquali SK, He X, Mohamad Z, McCrindle BW, Newburger JW, Li JS, et al. Trends in endocarditis hospitalizations at US children’s hospitals: impact of the 2007 American Heart Association antibiotic prophylaxis guidelines. Am Heart J. 2012;163(5):894-9.



