ABSTRACT
Although laser treatment has generated considerable interest among dentists and the public, there is no evidence that any laser system adds clinical value over and above scaling and root planing and conventional surgical treatment for periodontitis. Following a brief explanation of the mechanism behind soft tissue lasers, the evidence on the use of laser therapy in addition to traditional nonsurgical periodontal treatment in the management of periodontal diseases is reviewed.
Introduction
Recent publicity about the benefits of lasers in dentistry has generated considerable interest among dental professionals and the public. Lasers have been around for nearly 50 years.1 In 1985, they were introduced in a dental setting with the use of a modified ophthalmic laser.2 Some lasers have been designed for caries removal, while others are specific for soft tissues and have been used because of their hemostatic properties. More recently, lasers have been promoted as an adjunct to, or substitute for, standard mechanical debridement of subgingival root surfaces and periodontal pockets. The integration of dental lasers into the daily clinical practice of general dentists is being advocated as a “revenue booster,” offering patients a painless alternative to surgical treatment of periodontal disease and a benefit over traditional methods of therapy. Although it may be true that dental lasers can increase revenue, statements relating to the effects of lasers appear to be based primarily on manufacturers’ claims of laser efficacy rather than research data.
Some practitioners find it essential to have the most up-to-date technology available for their patients. Others may be more cautious, incorporating new therapy modalities only when evidence is strong enough to support their use. Clinicians should ask a number of questions before rushing out to buy the latest dentistry “gadget.” Is this new piece of equipment, material or treatment more effective than what is used now? If the effect is similar, are there other benefits that should be taken into account, such as a reduction in the amount of time to complete a procedure or reduced cost to the patient? What are the risks and side effects, and are they sufficient to offset the benefits?
This paper presents the most current clinical evidence on the use of soft tissue lasers in the nonsurgical treatment of patients with periodontal diseases to help clinicians with this decision.
How Lasers Work
The term laser is an acronym for “light amplification by stimulated emission of radiation.” Each type of laser device emits energy at a specific wavelength. For example, the wavelength emitted by diode (gallium:arsenide) lasers ranges from 635 to 950 nm, that of carbon dioxide (CO2) lasers is 10 600 nm. Radiation is delivered as a continuous, pulsed or running pulse waveform. The photons that make up the energy beam are emitted as coherent (in phase), unidirectional, monochromatic light, which is collimated into an intensely focused beam. As laser beams are in the infrared range, they are not visible; thus a quartz fibre channelling a red light is incorporated into the device to act as an aiming beam.3
When the beam is directed at target tissue, it may be absorbed, reflected or scattered.4 In biologic tissues, the energy is primarily absorbed; scattering occurs only with deep tissue penetration. Although the wavelength is the primary variable determining the extent of energy absorption, the optical properties of the target tissue are also an important determinant. Periodontal tissues are complex and vary with respect to water and mineral content, pigment and tissue density; thus, they also vary with respect to optical properties. Neodymium:yttrium-aluminium-garnet (Nd:YAG) and diode lasers are preferentially absorbed by pigmented tissues. The CO2 laser is well suited for soft tissue surgery, as its energy is highly absorbed by water. Other lasers are absorbed well by hydroxyapatite. Additional parameters affecting absorption include power, pulse duration, duration of exposure, angle of energy delivery and waveform (i.e., pulsed or continuous).
Energy absorption will cause the target tissue to react in 1 of 4 ways: warm up, coagulate, vaporize or, in the case of hard tissue, melt and recrystallize. Therefore, specific clinical treatment goals must be kept in mind when selecting the technology (laser or otherwise) best suited to achieve the desired outcomes.
Soft Tissue Lasers
The types of lasers most commonly used for periodontal applications are the diode, CO2, Nd:YAG and erbium:yttrium-aluminium-garnet (Er:YAG) (Table 1). All but 1 transmit the laser energy through an optical fibre, allowing the use of a handpiece and contact to provide tactile feedback. The CO2 laser does not have fibre optic delivery, but rather uses a light beam directly to guide the operator. However, because of the dry field of operation, visual feedback to the clinician is reportedly good.4 Recently, a 655-nm indium gallium arsenide phosphate diode laser has been added to the Er:YAG device to induce fluorescence in subgingival calculus. This feedback system purportedly improves the ability to remove calculus with minimal heat transfer to the root surface.5,6
Table 1 Use of lasers in soft tissue surgery
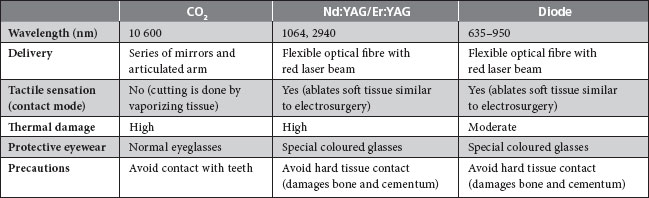
One of the primary advantages of laser therapy over conventional scalpel surgery is its superior hemostasis. Lasers have been used for a number of types of soft tissue surgeries, including gingivectomy, gingivoplasty, frenectomy, gingival troughing for impression taking and soft tissue biopsies. Patient acceptance of laser therapy is reportedly good.7 Patients often perceive laser therapy as a contemporary, progressive, more conservative and less painful approach than surgery. However, although the patient’s wishes should not be discounted, neither should they overrule the weight of scientific evidence.
Although there are reports that laser soft tissue wounds heal faster and produce less scar tissue than those from scalpel surgery, this is not borne out in the literature, in either histologic studies or clinical trials. In fact, studies of CO2 lasers report that healing is initially slower than after scalpel surgery.8 In addition, the activity of fibroblasts, the cells responsible for producing new connective tissue attachment in wound healing, is significantly delayed after exposure to Nd:YAG lasers.9
Lasers have been promoted for lengthening clinical crowns for esthetic and prosthetic reasons, without gingival flap reflection. However, no randomized controlled trials or cohort studies support the use of lasers for closed-flap crown lengthening. Further, this technique raises several questions. For example, without visual access to the underlying bone, how can the clinician determine the need for, or amount of, bone removal to establish proper anatomical dimensions to prevent invasion of the biologic width? Is there sufficient tactile sensitivity to allow the operator to distinguish between bone and cementum in a closed-flap technique? Results from studies of the effect of lasers on bone and root cementum have been mixed. Thermal side effects—melting, cracking and carbonization of the root—have been reported with the use of CO2 and Nd:YAG lasers.10,11
Reports in medical and veterinary journals comparing lasers and scalpels with respect to swelling, pain and wound healing have found that lasers may result in better outcomes.3 However, it is difficult to apply this information to dental situations, as most of these trials use a power setting 5 to 12 times that used in the oral cavity.12 Furthermore, the tissues primarily studied have been dermis and muscle distant from underlying bone.
Nonsurgical Periodontal Therapy
The use of lasers as an adjunct or alternative to conventional mechanical therapy is based on the claim that subgingival curettage and eradication of pathogenic bacteria will produce a sterile field, leading to elimination of periodontal pockets.
Subgingival curettage, with or without a dental laser, was originally designed to promote new connective tissue attachment to the root by removing diseased pocket lining.13 It has often been performed in conjunction with scaling and root planing (SRP), as a closed procedure. In other words, it does not allow better access for debridement or the improved visibility needed to achieve complete mechanical removal of plaque, calculus and bacterial biofilm. Research has shown that, regardless of the method used (lasers, ultrasonics or hand instruments), curettage has no additional benefit over SRP alone and, thus, has no justifiable application during active therapy for chronic periodontitis.14
The evidence supporting the claim that lasers sterilize periodontal pockets15 and, therefore, promote reattachment of previously diseased connective tissue to root surfaces is equally weak. Reduction in periodontal pathogens in and of itself is an insufficient measure of success. The gold standard in determining efficacy of nonsurgical periodontal therapy is gain in clinical attachment level. Although there is good evidence that laser energy can reduce or eliminate bacterial plaque, probing depths and levels of subgingival microbes are important only if they are associated with an increase in the degree of clinical attachment.
Photodynamic therapy is another purported use of lasers in nonsurgical periodontal therapy, based on the premise that excitation of photosensitive dyes by light promotes destructive action in biological systems. Bacteria are killed by visible light (i.e., lasers) in the presence of a sensitizing dye. The primary use of photodynamic therapy is as an alternative to chemotherapy or radiotherapy. In July 2009, a simple search of PubMed using the search terms “periodontal diseases”[MAJR] AND “photodynamic” with limits of “human,” “abstract” and “clinical trial” resulted in 20 articles. Despite the search limits, 9 were animal studies, 4 bench-top (in vivo) studies,1 interview and 1 study of endodontic lesions. Among the 4 randomized controlled clinical trials,16-19 all had a very small sample size and none found a clinically significant difference between SRP alone and SRP with the addition of photodynamic therapy. A review article20 had been published before all but 1 of the clinical trials. Although this does not constitute a thorough, systematic search of the literature, it illustrates the limitations of the clinical evidence supporting photodynamic therapy.
There is a striking dearth of high-quality clinical research examining the effect of laser use in nonsurgical debridement to improve periodontal outcomes, with or without adjunctive photodynamics. A comprehensive review3 commissioned for the American Academy of Periodontology located 278 articles on the use of lasers in periodontics published before 2006. Fewer than 10% were longitudinal or randomized controlled clinical trials (n = 20 and 3, respectively). Most of the articles (32%) were reviews.
More recently, 2 systematic reviews of the literature identified 19 randomized controlled clinical trials (Table 2). Schwarz and colleagues12 examined 11 studies of the clinical effects of laser therapy compared with mechanical debridement in patients with chronic periodontitis. One study compared the effect of a combination of CO2 and Nd:YAG laser monotherapy with that of ultrasonic scaling. Three reported the effects of diode laser in addition to hand instrumentation. A further 7 examined Er:YAG therapy, with and without feedback systems (and using a variety of fibre tips and energy settings). None of these trials found laser therapy—alone or as an adjunct to SRP—improved periodontal outcomes compared with SRP alone. There were no statistically significant differences in microbial levels, attachment gain, bleeding indices or pocket depth reduction in 10 of the 11 studies. In the 1 study that demonstrated a greater attachment level gain after laser therapy plus mechanical debridement, the difference (< 0.5 mm) was not clinically significant.
Table 2 Summary of randomized controlled clinical trials comparing lasers and mechanical debridement in the treatment of chronic periodontitisa
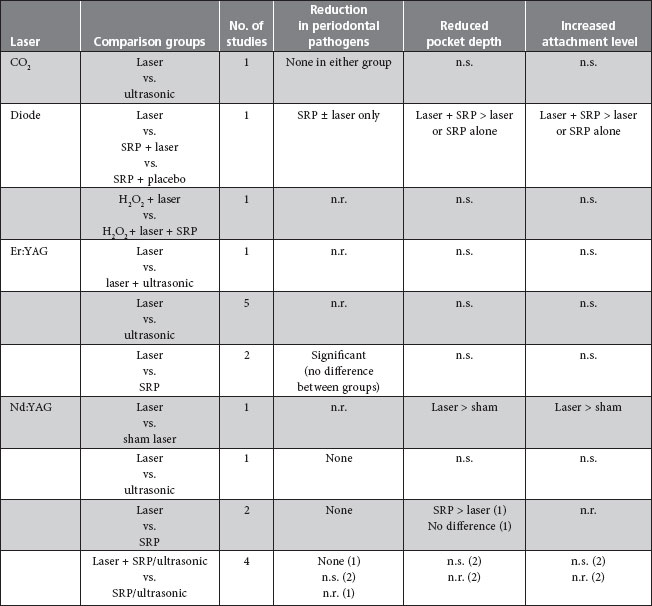
aAdapted from Slot et al.9 and Schwarz et al.12
Note: n.r. = not reported or insufficient data reported, n.s. = no significant difference between groups, SRP = scaling and root planing.
Most of these studies did not document adverse effects. Four reports on Er:YAG laser therapy reported postoperative healing for both the laser and nonlaser groups as uneventful. In 1 study, patients preferred ultrasonic treatment over laser instrumentation. Another reported no difference in postoperative pain between SRP and laser therapy.
Slot and colleagues9 reviewed 8 studies of the additional effect of pulsed Nd:YAG laser in nonsurgical periodontal therapy. Of these, 2 studies compared the Nd:YAG laser alone with SRP; 1 compared lasers with ultrasonic instrumentation and 1 with sham therapy (inserting the fibre tip into the pocket without irradiation); 4 compared laser therapy plus SRP with SRP alone. In 1 study, 4 groups measured the effect of order of treatment (laser alone, laser followed by SRP, SRP followed by laser and SRP alone). None of the studies found that laser therapy was more effective than traditional instrumentation with ultrasonic or hand instruments in terms of plaque reduction, pocket reduction, decreased bleeding or gain in clinical attachment levels.
Even though lasers received United States Food and Drug Administration clearance for soft tissue removal (as in gingivectomy/gingivoplasty), approval does not apply to the treatment of bacterially induced chronic periodontal diseases. It is important to note that Canada’s Food and Drugs Act regulates medical devices by a different set of standards than new pharmaceutical products, for example. This act is concerned with consumer safety and compliance with ISO standards, rather than efficacy.
Conclusion
Although there appear to be many claims surrounding the use of laser therapy in addition to or in place of traditional therapy, there is no evidence that any laser system adds clinical value over and above SRP and conventional surgical treatment. No long-term clinical studies have shown that laser therapy alone can be used effectively to treat adult chronic periodontitis. Such therapy does, however, add to patient cost for periodontal therapy.
THE AUTHOR
References
- Maiman TH. Stimulated optical radiation in ruby. Nature. 1960;187:493-4.
- Myers TD, Myers WD. In vivo caries removal utilizing the YAG laser. J Mich Dent Assoc. 1985;67(2):66-9.
- Cobb CM. Lasers in periodontics: a review of the literature. J Periodontol. 2006;77(4):545-64.
- Dederich DN, Bushick RD; ADA Council on Scientific Affairs and Division of Science; Journal of the American Dental Association. Lasers in dentistry: separating science from hype. J Am Dent Assoc. 2004;135(2):204-12. Erratum: J Am Dent Assoc. 2004;135(6):726-7.
- Eberhard J, Ehlers H, Falk W, Acil Y, Albers HK, Jepsen S. Efficacy of subgingival calculus removal with Er:YAG laser compared to mechanical debridement: an in situ study. J Clin Periodontol. 2003;30(6):511-8.
- Krause F, Braun A, Brede O, Eberhard J, Frentzen M, Jepsen S. Evaluation of selective calculus removal by a fluorescence feedback-controlled Er:YAG laser in vitro. J Clin Periodontol. 2007;34(1):66-71. Epub 2006 Nov 27.
- Ishikawa I, Aoki A, Takasaki AA, Mizutani K, Sasaki KM, Izumi Y. Application of lasers in periodontics: true innovation or myth? Periodontol 2000. 2009;50:90-126.
- Lippert BM, Teymoortash A, Folz BJ, Werner JA. Wound healing after laser treatment of oral and oropharyngeal cancer. Lasers Med Sci. 2003;18(1):36-42.
- Slot DE, Kranendonk AA, Paraskevas S, Van der Weijden F. The effect of a pulsed Nd:YAG laser in non-surgical periodontal therapy. J Periodontol. 2009;80(7):1041-56.
- Tucker D, Cobb CM, Rapley JW, Killoy WJ. Morphologic changes following in vitro CO2 laser treatment of calculus-ladened root surfaces. Lasers Surg Med. 1996;18(2):150-6.
- Israel M, Cobb CM, Rossmann JA, Spencer P. The effects of CO2, Nd:YAG and Er:YAG lasers with and without surface coolant on tooth root surfaces. An in vitro study. J Clin Periodontol. 1997;24(9 Pt 1):595-602.
- Schwarz F, Aoki A, Becker J, Sculean A. Laser application in non-surgical periodontal therapy: a systematic review. J Clin Periodontol. 2008;35(8 Suppl):29-44.
- Goldman HM. Subgingival curettage: a rationale. J Periodontal Res. 1948;19(2):54-62.
- Caton JG, Zander HA. The attachment between tooth and gingival tissues after periodic root planing and soft tissue curettage. J Periodontol. 1979;50(9):462-6.
- Midda M, Renton-Harper P. Lasers in dentistry. Br Dent J. 1991;170(9):343-6.
- Braun A, Dehn C, Krause F, Jepsen S. Short-term clinical effects of adjunctive antimicrobial photodynamic therapy in periodontal treatment: a randomized clinical trial. J Clin Periodontol. 2008;35(10):877-84. Epub 2008 Aug 17.
- Christodoulides N, Nikolidakis D, Chondros P, Becker J, Schwarz F, Rossler R, et al. Photodynamic therapy as an adjunct to non-surgical periodontal treatment: a randomized, controlled clinical trial. J Periodontol. 2008;79(9):1638-44.
- de Oliveira RR, Schwartz-Filho HO, Novaes AB Jr, Taba M Jr. Antimicrobial photodynamic therapy in the non-surgical treatment of aggressive periodontitis: a preliminary randomized controlled clinical study. J Periodontol. 2007;78(6):965-73.
- de Oliveira RR, Schwartz-Filho HO, Novaes AB, Garlet GP, de Souza RF, Taba M, et al. Antimicrobial photodynamic therapy in the non-surgical treatment of aggressive periodontitis: cytokine profile in gingival crevicular fluid, preliminary results. J Periodontol. 2009;80(1):98-105.
- Meisel P, Kocher T. Photodynamic therapy for periodontal diseases: state of the art. J Photochem Photobiol B. 2005;79(2):159-70.
