ABSTRACT
Disease related to peri-implant mucosal inflammation (PIMI) has been reported as one of the major factors leading to failure of dental implants. Many authors have suggested options for treatment of these lesions, ranging from simple mechanical debridement and regenerative surgical techniques to removal of the implant. Prognostic classification systems have become an integral part of dental practice because they provide direct guidance in planning treatment. However, there is limited evidence to support the appropriate classification of PIMI and the corresponding treatments, and a more specific prognostic system is required. Because the number of patients with implants is increasing, clinicians must be aware of PIMI as an entity with specific management requirements. This report describes a simple prognostic system to help clinicians to foresee the outcomes of PIMI treatment.
Introduction
Peri-implant diseases are characterized by inflammatory lesions that may affect just the peri-implant mucosa (mucositis) or that may also result in loss of the supporting bone (peri-implantitis). This loss of bone can in turn lead to loss of the implant. Peri-implant mucositis occurs in about 80% of patients who have undergone placement of implants (at 50% of implant sites), whereas peri-implantitis occurs in 28% to 56% of patients (at 12% to 40% of implant sites).1-4 Several risk factors have been identified, including poor oral hygiene, history of periodontitis, diabetes mellitus and smoking.5 The diagnosis of peri-implant disease requires the use of probing techniques to identify the presence or absence of bleeding, pain and suppuration, all of which indicate clinical inflammation.5 Radiographs are also required to detect loss of supporting bone. Diagnostic information should be obtained for all implant patients once placement and healing of the implant is complete, to allow for longitudinal monitoring of peri-implant conditions.
Articles about the causes and treatment of peri-implant disease are now being published.5-8 Authors have claimed that proposed anti-infective therapies can modulate local inflammation and improve clinical parameters for the peri-implant tissues.7 Mechanical debridement combined with antiseptic or antibiotic therapy, Er:YAG (erbium-doped yttrium aluminum garnet) laser and regenerative techniques have been used to treat peri-implantitis, but the indications for each of these techniques have not been clearly delineated.5,8 Assessment of the real effect of therapy is particularly important when complex interventions and expensive materials are used. For example, a patient undergoing treatment for peri-implantitis with bone substitutes plus membranes (guided bone regeneration) should be clearly informed about the potential gains. Specifically, the patient should be told that the potential additional improvements in terms of surrogate end points such as probing depth and clinical attachment level resulting from the surgical approach, relative to noninvasive procedures, do not guarantee long-term retention of the implant.8
According to recent systematic reviews,9,10 studies of treatment options for peri-implant inflammation have generally been limited in number, with small sample sizes and short follow-up periods. Also, the reviews have not revealed whether therapy for peri-implantitis has been useful in reducing the risk of implant failure.8,10 Finally, there have been substantial differences between human and animal studies in terms of study design and treatment procedures.10
Oral health care providers have not yet used a prognostic system to guide the treatment of peri-implant mucosal inflammation (PIMI), considered here as a condition separate from, albeit related to, peri-implantitis. It seems reasonable to assume that a prognostic system for PIMI could be based on the probability of stabilizing the peri-implant tissues, rather than the prospect of implant failure. Therefore, given the general acceptability of prognostic systems11 and the availability of supportive therapy for periodontal diseases, a system was developed for determining the prognosis in cases of PIMI and selecting appropriate supportive implant therapy.12,13
Importance of PIMI
Various nonsurgical and surgical approaches for the management or treatment of PIMI and related conditions have been described,12-14 but they do not necessarily represent a prognostic system or a protocol for supportive implant therapy. Information is also lacking about how implants, both healthy ones and those affected by PIMI, alter the local and systemic inflammatory response in short- and long-term prognostic approaches. There is no reason to believe that mucosal inflammation affecting endosseous implants (i.e., PIMI) would have fewer effects on general health than similar levels of inflammation affecting the teeth (e.g., periodontitis, gingivitis).
Prognostic Algorithm
The algorithm proposed here (Fig. 1) offers oral health care providers a rational approach to determining the prognosis for a PIMI lesion, as well as indicating possible treatment options and a protocol for supportive implant therapy. The algorithm includes surgical modalities7 for the management of certain presentations of PIMI and also less invasive approaches, such as oral hygiene methods. Just as a protocol for supportive periodontal therapy is expected to reduce inflammation in the periodontal tissues, so a protocol for supportive implant therapy should reduce inflammatory diseases associated with implants. Furthermore, if PIMI has effects on general health that are similar to those of periodontal inflammation, then the management and control of PIMI could lead to improvements in biomarkers15 that predict and regulate general health. The system for recall appointments is based on existing knowledge for periodontal maintenance.
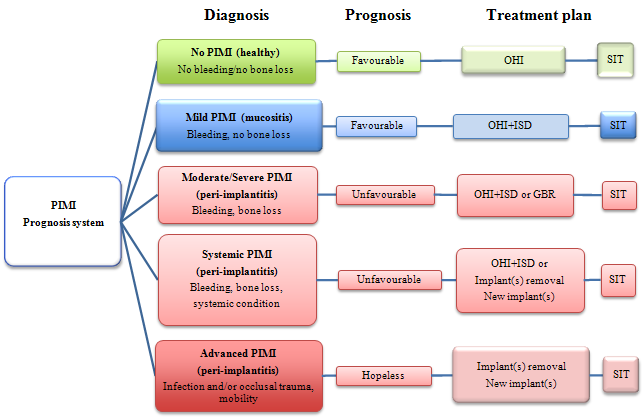 Figure 1: Algorithm for determining prognosis of and appropriate treatment for dental implants with peri-implant mucosal inflammation (PIMI). GBR = guided bone regeneration, ISD = implant surface debridement, OHI = oral hygiene instruction, SIT = supportive implant therapy.
Figure 1: Algorithm for determining prognosis of and appropriate treatment for dental implants with peri-implant mucosal inflammation (PIMI). GBR = guided bone regeneration, ISD = implant surface debridement, OHI = oral hygiene instruction, SIT = supportive implant therapy.
Extent and Impact of PIMI
The impact of and relationships between periodontal health and diseases involving other organs and physiologic systems have been established16-20 with increasing reliability. The notion of a potential influence of PIMI on general health and disease has been discussed only recently.21 There is no evidence that inflammatory disease in the gingival tissues surrounding endosseous implants predisposes patients to other systemic diseases (e.g., cardiovascular disease). Similarly, it is not yet known whether PIMI exacerbates other conditions such as diabetes mellitus, but the model of periodontal disease strongly suggests this possibility.21
Multiyear success rates above 90% have been reported for most implant systems for both fully and partially edentulous patients.3 However, it is becoming increasingly clear that despite this level of success, osseointegrated implants are susceptible to disease conditions that may lead to peri-implant inflammatory disease or, ultimately, failure of the implant.22 Indeed, peri-implant infections are thought to be the major cause of these later failures.23,24 However, it must also be recognized that the long-term success of a dental implant is largely determined by its location,25 with an apparently higher success rate for implants in the anterior region than for those in the posterior region. This is due in part to the quality of the bone, there being a quality difference of about 12% between the anterior and posterior maxillae and a difference of about 4% between the anterior and posterior mandibles.25 As such, treatment of implants in the anterior mandible is the most successful, whereas treatment of implants in the posterior maxilla is the least successful.25 Similar disease patterns have been reported for periodontitis, which implies that the knowledge base for periodontitis and its effect on general health and disease presentation could well apply to implant-related diseases.
PIMI and Implant Failure: An Imminent Tidal Wave
Implants have become increasingly popular since their endorsement by the American Dental Association in 1986. The average number of implants placed surgically by dentists who perform the procedure increased by 49% over a 4-year period (from 37.7 annually per dentist in 1995 to 56.2 annually in 1999).21 It is estimated that more than 400 000 implants are placed every year in the United States.26 Recent findings from Europe indicate that every year about 120 000 implants are placed in France, 185 000 in Spain, 410 000 in Italy and 420 000 in Germany.21 Data on the number of implants placed annually in Canada are not available.
The failure rate of dental implants varies according to the type of system evaluated, as well as by the type of study. One recent publication indicated a rate of 2.73%,27 a figure that will undoubtedly increase over time, given that the use of endosseous oral implants has become increasingly routine in a relatively short period.
In summary, it appears that a “tidal wave” of ailing and/or failing implants may be imminent. Apart from the implications of this problem in terms of the growing need for clinical treatment, the possibility of a relationship between PIMI and general health and disease means that we are also facing a potential increase in the incidence and/or severity of “non-oral” disorders.21 Despite these considerations, it is still unclear whether every failing implant has had previous PIMI, occlusal trauma or both.28 The algorithm for prognosis and treatment of endosseous implant diseases (Fig. 1) suggests that patients with non-oral disorders who also have dental implants (including implants that appear healthy) may have more inflammation (both oral and systemic) than patients with healthy dentition or those who are completely edentulous. For the purposes of the algorithm, it was essential to define parameters clearly and to describe the various potential severities or presentations of this condition within the clinical milieu.
Determining Prognosis and Related PIMI Conditions
Determining the prognosis of a disease or condition is an evolving and dynamic process.11 Therefore, in cases of PIMI, periodic reassessment of the prognosis is desirable. The prognosis may change over time, and PIMI may recur after initial treatment. As such, it may be advisable to repeat the prognostic exercise at each recall appointment.
An assessment of pain is one aspect of the algorithm. A simple visual analogue scale (VAS) has been suggested for determining the patient’s perception of pain. The VAS is a horizontal line, 10 cm in length, anchored by verbal descriptors of the extremes of pain at each end, with the least amount of pain at the left end. The patient marks on the line the point representing his or her perception of the current state of pain. The numeric VAS score is determined by measuring the distance from the left-most end of the line to the point marked by the patient.
The following sections list the characteristics suggested for each prognostic category of PIMI.
Overall Favourable Outcomes
- Healthy Implant (No PIMI): No bleeding or pain on probing (regardless of probing depth), no suppuration, no implant-related halitosis, no radiographic bone loss, apart from the usual loss near microgap areas, no occlusal trauma or mobility, no erythema in surrounding soft tissues.
- Mild PIMI: Bleeding on probing, pain on probing (1–3 cm on a VAS for pain), mild erythema of surrounding soft tissues, no suppuration, no radiographic bone loss, no occlusal trauma or mobility, no impact on systemic markers of inflammation.
Overall Unfavourable Outcomes
- Moderate PIMI: Bleeding on probing, pain on probing (3–6 cm on a VAS for pain), generalized erythema of soft tissues surrounding the implant, possible suppuration, radiographic evidence of early bone loss (i.e., exposure of 3 or more threads, bone loss < 50%), no occlusal trauma or mobility, mild elevation (10%–20%) of systemic markers of inflammation.
- Severe PIMI: Bleeding on probing, pain on probing (6–10 cm on a VAS for pain), generalized erythema of soft tissues surrounding the implant, possible suppuration, radiographic evidence of moderate bone loss (i.e., exposure of 5 or more threads, bone loss > 50%), possible occlusal trauma, no mobility, moderate elevation (> 20%) of systemic markers of inflammation.
- Systemic PIMI: All characteristics of severe PIMI (described above), along with complicating systemic, genetic and/or environmental conditions (e.g., smoking, diabetes mellitus, bisphosphonate therapy, radiation). May also be seen in patients with previously diagnosed refractory periodontitis.29
Overall Hopeless Outcomes
- Advanced PIMI: Bleeding on probing, pain on probing (6–10 cm on a VAS for pain), generalized erythema of soft tissues surrounding the implant, probable suppuration and occlusal trauma, moderate to severe radiographic evidence of bone loss (> 50%, with “trough” visible around the implant) leading to loss of osseointegration and development of implant mobility, moderate increase (> 20%) in systemic markers of inflammation. Usually leads to loss of implant.
Rationale for Treatment Modalities
- Healthy Implant (No PIMI):There is evidence that a rational approach to maintaining healthy implants should include instruction in basic oral hygiene.8 The patient should comply with supportive implant therapy and should attend regular recall visits.14,23
- Mild PIMI: There is evidence that a rational approach to the treatment of mild PIMI should include instruction in basic oral hygiene combined with eventual removal of calculus on the abutment surfaces by means of plastic scalers and/or ultrasonic debridement.29-32 The patient should comply with supportive implant therapy and should attend regular recall visits.14,23
- Moderate PIMI: Treatment of moderate PIMI should incorporate instruction in basic oral hygiene and surface debridement of the implant in the area of peri-implant pockets by means of plastic scalers and/or ultrasonic debridement (possibly under local anesthesia).7 If there is no response to these approaches, an apical flap can be created, with exposure of the implant threads, to improve local control of dental biofilm and to improve oral hygiene; alternatively, guided bone regeneration may be performed. If these measures are unsuccessful, the implant must be considered as failing and should be replaced.12,14 A patient with moderate PIMI should comply with supportive implant therapy and should attend regular recall visits.14,23
- Severe PIMI: The treatment of severe PIMI is similar to the treatment of moderate PIMI, incorporating instruction in basic oral hygiene, along with surface debridement of the implant in the area of peri-implant pockets by means of plastic scalers and/or ultrasonic debridement (possibly under local anesthesia).7 If there is no response, an apical flap can be created to expose the implant threads, to improve local control of dental biofilm and to improve oral hygiene. Because bone loss in this category is greater than with moderate PIMI, a decision will be needed to perform guided bone regeneration or to consider the implant as failing, in which case it should be replaced.12,14 A patient with severe PIMI should comply with supportive implant therapy and should attend regular recall visits.14,23
- Systemic PIMI: In cases of peri-implantitis associated with environmental and/or systemic conditions, it is unlikely that satisfactory control of PIMI will be established unless satisfactory management of the environmental or systemic condition can be achieved.33,34 If smoking is deemed to be a factor in the PIMI, there should be more emphasis than usual on the benefits of smoking cessation.35 Current evidence suggests that the most rational approach to this form of PIMI includes instruction in basic oral hygiene, debridement of the implant surface, creation of an apical flap with exposure of threads (as described above) and, ultimately, implant replacement. Guided bone regeneration techniques should be considered in cases of unstable systemic conditions. The clinician should be alert to the possibility of a “cluster phenomenon” whereby failure of one implant increases the patient’s risk of losing additional implants (which would suggest a systemic contributing factor). The patient should comply with supportive implant therapy. Because the systemic condition may be affecting the patient’s overall immunologic stability,15,17,21 recall visits should be more frequent14,23 so as to prevent eventual loss of additional implants and natural teeth.
- Advanced PIMI: This PIMI category is essentially a terminal condition insofar as implant retention is concerned. Most evidence suggests that advanced PIMI most likely represents peri-implantitis causing mobility. In these cases, the implant should be replaced and appropriate bone augmentation approaches undertaken. Any patient who has had or could develop peri-implantitis in association with another implant should comply with supportive implant therapy, with regular maintenance visits.14,23
Conclusions
In light of the foregoing discussion, determining the prognosis for peri-implant diseases may seem audacious. However, the proposed prognostic system is based on the projected stability of the peri-implant tissues and loss of the implant (i.e., surrogate and true end points) and thus may foster the ultimate development of a more logical classification for the treatment of PIMI. The concurrent establishment of a suitable protocol for supportive implant therapy also requires additional basic and clinical research to better understand the patterns of disease development and to define the appropriate care for PIMI in healthy patients and those with systemic compromise.
The prognostic algorithm for PIMI (Fig. 1) could also be used as a framework for educating dentists and dental hygienists, so that they can provide more appropriate care for implants with associated mucosal inflammatory disease. The implementation of a reliable prognostic protocol could lead to reductions in costs and improvements in patient benefits related to the placement and maintenance of implants. The proposed prognostic system should be adopted in clinical practice to verify its validity and usefulness.
THE AUTHORS
References
- Lemmerman KJ, Lemmerman NE. Osseointegrated dental implants in private practice: a long-term case series study. J Periodontol. 2005;76(2):310-9.
- Attard NJ, Zarb GA. Long-term treatment outcomes in edentulous patients with implant-fixed prostheses: the Toronto study. Int J Prosthodont. 2004;17(4):425-33.
- Lambrecht JT, Filippi A, Künzel AR, Schiel HJ. Long-term evaluation of submerged and nonsubmerged ITI solid-screw titanium implants: a 10-year life table analysis of 468 implants. Int J Oral Maxillofac Implants. 2003;18(6):826-34.
- Brånemark PI, Adell R, Breine U, Hansson BO, Lindstrom J, Ohlsson A. Intra-osseous anchorage of dental prostheses. I. Experimental studies. Scand J Plast Reconstr Surg. 1969;3(2):81-100.
- Lindhe J, Meyle J, Group D of European Workshop on Periodontology. Peri-implant diseases: Consensus Report of the Sixth European Workshop on Periodontology. J Clin Periodontol. 2008;35(8 Suppl):282-5.
- Renvert S, Samuelsson E, Lindahl C, Persson GR. Mechanical non-surgical treatment of peri-implantitis: a double-blind randomized longitudinal clinical study. I: clinical results. J Clin Periodontol. 2009;36(7):604-9. Erratum in: J Clin Periodontol. 2009;36(12):1076.
- Duarte PM, de Mendonça AC, Máximo MB, Santos VR, Bastos MF, Nociti FH. Effect of anti-infective mechanical therapy on clinical parameters and cytokine levels in human peri-implant diseases. J Periodontol. 2009;80(2):234-43.
- Faggion CM Jr, Listl S, Tu YK. Assessment of endpoints in studies on peri-implantitis treatment—a systematic review. J Dent. 2010;38(6):443-50. Epub 2010 Mar 11. Review.
- Kotsovilis S, Karoussis IK, Trianti M, Fourmousis I. Therapy of peri-implantitis: a systematic review. J Clin Periodontol. 2008;35(7):621-9. Epub 2008 May 11.
- Faggion CM Jr, Chambrone L, Gondim V, Schmitter M, Tu YK. Comparison of the effects of treatment of peri-implant infection in animal and human studies: systematic review and meta-analysis. Clin Oral Implants Res. 2010;21(2):137-47. Epub 2009 Nov 13.
- Kwok V, Caton JG. Commentary: prognosis revisited: a system for assigning periodontal prognosis. J Periodontol. 2007;78(11):2063-71.
- Renvert S, Polyzois I, Maguire R. Re-osseointegration on previously contaminated surfaces: a systematic review. Clin Oral Implants Res. 2009;20 Suppl 4:216-27.
- Karoussis IK, Salvi GE, Heitz-Mayfield LJ, Brägger U, Hämmerle CH, Lang NP. Long-term implant prognosis in patients with and without a history of chronic periodontitis: a 10-year prospective cohort study of the ITI Dental Implant System. Clin Oral Implants Res. 2003;14(3):329-39.
- Interventions for replacing missing teeth: maintaining and re-establishing healthy tissues around dental implants. Cochrane Database Syst Rev. 2002;(3):CD003069. Review.
- Guncu GN, Tozum TF, Guncu MB, Yamalik N. Relationships between implant stability, image-based measures and nitric oxide levels. J Oral Rehabil. 2008;35(10):745-53. Epub 2008 Apr 14.
- Hein C. Translating evidence of oral-systemic relationships into models of interprofessional collaboration. J Dent Hyg. 2009;83(4):188-9. Epub 2009 Nov 2.
- Hein C. Scottsdale revisited: the role of dental practitioners in screening for undiagnosed diabetes and the medical co-management of patients with diabetes or those at risk for diabetes. Compend Contin Educ Dent. 2008;29(9):538-40, 542-4, 546-53.
- Iacopino AM. What is the role of inflammation in the relationship between periodontal disease and general health? J Can Dent Assoc. 2008;74(8):695.
- Iacopino AM. Practising oral-systemic medicine: the need for interprofessional education. J Can Dent Assoc. 2008;74(10):866-7.
- Iacopino AM. Periodontitis and diabetes interrelationships: role of inflammation. Ann Periodontol. 2001;6(1):125-37.
- Tenenbaum H, Glogauer M, Lanzberg M, Goldberg M. Systemic implications of peri-implant inflammation; mimicry of the periodontitis-systemic disease model? In: Asbjorn Jokstad, editor. Osseointegration and dental implants., Toronto: Wiley Press; 2008. p.74-84.
- Iacono VJ; Committee on Research, Science and Therapy, the American Academy of Periodontology. Dental implants in periodontal therapy. J Periodontol. 2000;71(12):1934-42.
- Chen S, Darby I. Dental implants: maintenance, care and treatment of peri-implant infection. Aust Dent J. 2003;48(4):212-20.
- Lang NP, Wilson TG, Corbet EF. Biological complications with dental implants: their prevention, diagnosis and treatment. Clin Oral Implants Res. 2000;11(Suppl 1):146-55.
- Tolstunov L. Implant zones of the jaws: implant location and related success rate. J Oral Implantol. 2007;33(4):211-20.
- What are implants? Aetna, Inc.; ©2002-2005. www.colgate.com/app/Colgate/US/OC/Information/OralHealthBasics/CheckupsDentProc/DenturesAndDentalImplants/WhatAreImplants.cvsp (accessed 2011 Jan 13).
- Popelut A, Valet F, Fromentin O, Thomas A, Bouchard P. Relationship between sponsorship and failure rate of dental implants: a systematic approach. PLoS One.2010;5(4):e10274.
- van Steenberghe D, Naert I, Jacobs R, Quirynen M. Influence of inflammatory reactions vs. occlusal loading on peri-implant marginal bone level. Adv Dent Res. 1999;13:130-5.
- Heitz-Mayfield LJ. Diagnosis and management of peri-implant diseases. Aust Dent J. 2008;53:(Suppl 1):S43–8.
- Humphrey S. Implant maintenance. Dent Clin North Am. 2006;50(3):463-478.
- Porras R, Anderson GB, Caffesse R, Narendran S, Trejo PM. Clinical response to 2 different therapeutic regimens to treat peri-implant mucositis. J Periodontol. 2002;73(10):1118-25.
- Greenstein G; Research, Science and Therapy Committee of the American Academy of Periodontology. Position paper: The role of supra- and subgingival irrigation in the treatment of periodontal diseases. J Periodontol. 2005;76(11):2015-27.
- Ferreira SD, Silva GL, Cortelli JR, Costa JE, Costa FO. Prevalence and risk variables for peri-implant disease in Brazilian subjects. J Clin Periodontol. 2006;33(12):929-35.
- Schou S. Implant treatment in periodontitis-susceptible patients: a systematic review. J Oral Rehabil. 2008;35(Suppl 1):9-22. Review.
- Sham AS, Cheung LK, Jin LJ, Corbet EF. The effects of tobacco use on oral health. Hong Kong Med J. 2003;9(4):271-7.
