Abstract
Reconstruction of large mandibular defects, whether involving the temporomandibular joint (TMJ) or not, has historically been achieved with autogenous grafts, such as free costochondral grafts and vascularized bone grafts. Ensuring intact, functioning microcirculation is critical for graft survival in the face of postoperative radiation therapy secondary to malignant tumour ablation. However, in the case of benign tumours, such as ameloblastomas, postoperative radiation therapy is not required, thus increasing the options for reconstruction. Alloplastic components coupled with nonvascularized bone grafts have been used successfully to restore mandibular form, function and esthetics after extensive mandibular resection. In this article, we describe a case of a multiply recurrent ameloblastoma treated by left hemimandibulectomy and immediate reconstruction with a custom-fabricated alloplastic system in combination with an anterior iliac crest bone graft. The result was a high degree of mandibular function and facial cosmesis, minimal donor-site morbidity, and nearly immediate return to function.
Ameloblastomas are slow-growing, locally invasive tumours arising from the odontogenic epithelium and lacking ectomesenchymal elements.1 Although these tumours exhibit a characteristic of malignancy, specifically infiltration of surrounding tissues, they are considered benign as they exhibit virtually no tendency to metastasize.1 Ameloblastomas represent 1% of all mandibular and maxillary tumours and cysts and approximately 10% of all odontogenic mandibular tumours.2,3 They may arise from remnants of the dental lamina, a developing enamel organ, the epithelial lining of an odontogenic cyst, or the basal cells of the oral mucosa.4-6
Treatment of ameloblastomas ranges from simple enucleation and curettage to en bloc resection, depending on the clinicoradiographic presentation of the tumour.7,8 Enucleation and curettage often leave small islands of tumour within bone, which may later manifest as recurrences or, more accurately, persistent tumour.9 After curettage alone, recurrence rates range from 50%–90%, sometimes as much as 30 years after initial treatment, making regular follow-up visits, even after 5-years, highly recommended.10 After marginal or en bloc resection, recurrence rates of up to 15% have been found; however, some tumours may not be amenable to this approach because of their size or growth pattern, e.g., intracranial extension of the tumour. Radiation therapy is seldom used because of the intraosseous location of these tumours and the potential for secondary radiation-induced malignancies.11 Although most ameloblastomas are not life threatening, death may very rarely result from their progressive spread to involve vital structures.
If a large segment of mandibular (or maxillary) bone is resected, the surgeon must arrange for immediate or delayed reconstruction. Reconstruction of large mandibular defects, whether involving the temporomandibular joint (TMJ) or not, has historically been achieved with autogenous grafts, such as free costochondral grafts12,13 and vascularized bone grafts.14,15 Ensuring intact, functioning microcirculation is critical for graft survival in the face of postoperative radiation therapy secondary to malignant tumour ablation. However, in the case of a benign tumour, such as an ameloblastoma, postoperative radiation therapy is not required, thus increasing the options for reconstruction. Alloplastic (prosthetic) components coupled with nonvascularized bone grafts have been used successfully to restore mandibular form, function and esthetics after extensive mandibular and TMJ resection.16
In this article, we describe a case of recurrent ameloblastoma treated by left hemimandibulectomy and immediate reconstruction with a custom-fabricated alloplastic system in combination with an anterior iliac crest bone graft.
Report of the Case
An otherwise healthy 55-year-old man was referred to the senior author with a recurrent lesion in the left posterior mandible. The patient had been experiencing increasing discomfort due to trismus and buccolingual swelling in the area of the lesion. The referring surgeon had removed the primary lesion, which was diagnosed histologically as unicystic ameloblastoma, intramural type. A subsequent recurrence had also been treated by enucleation and curettage. Thus, the patient was presenting with a second recurrence.
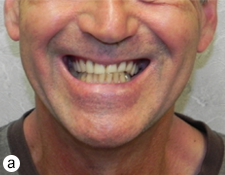
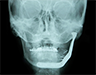
Extraoral examination revealed normal facial symmetry and no visible changes to the skin (Figs. 1a and 1b). There was no palpable cervical lymphadenopathy, and cranial nerves II through XII were grossly intact. Intraoral examination revealed fullness of the left posterior buccal vestibule and tenderness to palpation extending to the left coronoid process, but no discolouration of the overlying mucosa. A palpable buccolingual expansion of the left coronoid process was noted. The patient was edentulous in the maxilla, and partly dentate in the mandible. Maximum interincisal opening was limited to 33 mm.
NOTE: Click to enlarge images.
Figure 1: Clinical photographs of (a) frontal and (b) profile views show no noticeable swelling of the face.
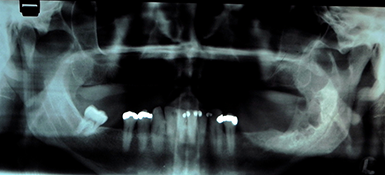
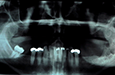
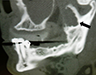
Panoramic tomography revealed a multilocular radiolucent lesion in the left mandibular ramus (Fig. 2). Computed tomography (CT) showed significant expansion of the medial and lateral cortices in the coronoid process region with perforation of the cortical plates. A soap-bubble, multilocular radiolucency could be appreciated (Fig. 3). Magnetic resonance imaging (MRI) demonstrated fluid within the lesion and no invasion into the surrounding soft tissues, despite cortical perforation.
A preoperative biopsy was not performed as the diagnosis of unicystic ameloblastoma, intramural type, had been made before the first 2 surgical efforts. However, the current radiographic presentation was suggestive of multicystic ameloblastoma.
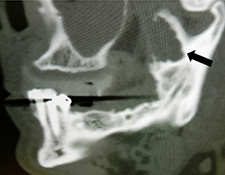
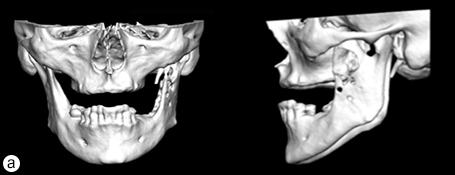
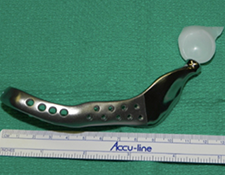
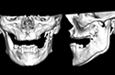
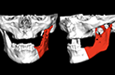
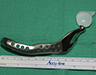
Because the presenting tumour was the second recurrence, a segmental resection of the left mandible, including 1.0–1.5 cm margins, was planned. Extension of the lesion from the left mandibular body to the coronoid process and the mandibular condyle necessitated a partial mandibulectomy posterior to the left mental foramen. Simultaneous mandibular and TMJ reconstruction was planned using 3-dimensional (3D) CT imaging and computer-assisted design (Medical Modeling, Denver, Colorado). The 3D CT imaging showed the lesion in the left native mandible (Fig. 4a) and the planned resection margins (Fig. 4b). Using a virtual model of the prosthesis, an alloplastic system composed of a metallic mandible–condylar component and a polyethylene glenoid fossa was custom manufactured (Fig. 5). The mandibular body portion of the prosthesis included screw holes for stabilization of an autogenous bone graft, which would facilitate later placement of dental implants.
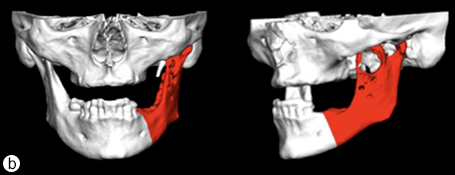 Figure 4: Three-dimensional imaging using CT data shows: (a) coronal and sagittal views of the lesion in the left angle, coronoid and subcondylar regions and (b) the planned resection margins (in red) posterior to the left mental foramen
Figure 4: Three-dimensional imaging using CT data shows: (a) coronal and sagittal views of the lesion in the left angle, coronoid and subcondylar regions and (b) the planned resection margins (in red) posterior to the left mental foramen
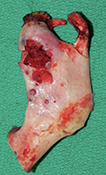
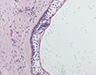
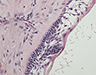
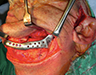
During the surgery, the lesion was accessed via submandibular, submental and preauricular incisions. As planned, the entire mandible posterior to the left mental foramen was resected (Fig. 6). After the inferior alveolar nerve (IAN) was partly decompressed, cut and released from the proximal mandible, the specimen was removed. First, the mandibular condyle was sectioned and retrieved through the preauricular incision, followed by removal of the mandibular body portion through the submandibular incision. Repair of the IAN was achieved by reanastomosis with microsuturing. The specimens were sent for histologic examination (Figs. 7a, b, c), and the final diagnosis was recurrent multicystic ameloblastoma.
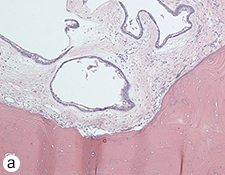
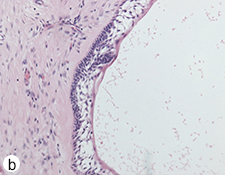
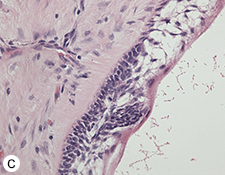 Figure 7: Morphologic features of the tumour stained with hematoxylin and eosin. (a) Solid and cystic components with epithelial aggregates in a background of fibrous tissue stroma and mature lamellar bone captured at 50X magnification. (b and c) Columnar basal epithelial cells showing reverse polarization typical of ameloblastomas captured at 100X and 200X magnification, respectively.
Figure 7: Morphologic features of the tumour stained with hematoxylin and eosin. (a) Solid and cystic components with epithelial aggregates in a background of fibrous tissue stroma and mature lamellar bone captured at 50X magnification. (b and c) Columnar basal epithelial cells showing reverse polarization typical of ameloblastomas captured at 100X and 200X magnification, respectively.
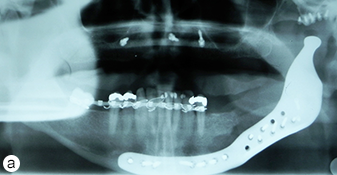
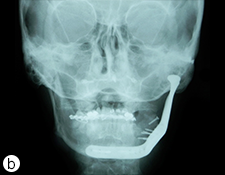
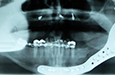
After harvesting a corticocancellous block graft from the left anterior iliac crest, the alloplastic system was installed. Insertion of the fossa component was followed by fixation of the mandibular component with screws in both the native mandible and the corticocancellous block graft (Fig. 8). Postoperative panoramic tomography (Fig. 9a) and anteroposterior cephalometric radiographs (Fig. 9b) revealed a smooth mandibular contour mirroring that of the right mandible.
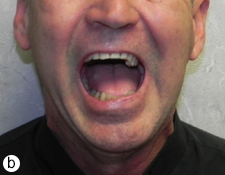
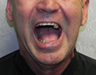
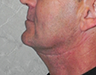
The postoperative course was uneventful. At short- and long-term follow-up visits, the patient showed good facial symmetry, pain control, chewing and mandibular function (Fig. 10). Sensory function in the lower lip and chin had returned to normal by 6-months after surgery.
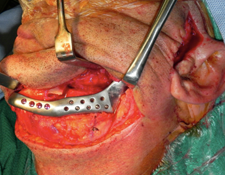 Figure 8: Fixation of the mandibular component of the alloplastic prosthesis was achieved by placing screws into both the distal mandible and the iliac crest bone graft.
Figure 8: Fixation of the mandibular component of the alloplastic prosthesis was achieved by placing screws into both the distal mandible and the iliac crest bone graft.
Figure 9: (a) Postoperative panoramic tomogram and (b) anteroposterior cephalometric radiograph show smooth mandibular contour.
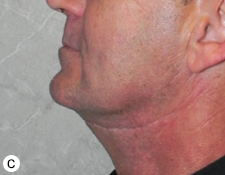 Figure 10: Photographs taken 16-months after surgery: frontal view with (a) mouth closed (b) mouth open and (c) profile view.
Figure 10: Photographs taken 16-months after surgery: frontal view with (a) mouth closed (b) mouth open and (c) profile view.
Discussion
Of all clinically significant odontogenic tumours, the ameloblastoma is the most common; however, it is unique in that it is potentially fatal if mismanaged. Historically, there has been controversy over the curative management of ameloblastomas. Each of the 3 variants, solid or multicystic, unicystic and peripheral, has specific implications for treatment and a unique prognosis. Numerous methods of treatment have been implemented, ranging from simple enucleation and curettage to resection.17-22
Conventional ameloblastoma presents as a painless swelling or expansion of the jaw, which, if left untreated, may slowly grow to massive proportions.23 Pain and paresthesia are uncommon even with large, infiltrative tumours as ameloblastomas generally do not invade the neurovascular bundle.24 Radiographic examination shows a multilocular radiolucent lesion with a “soap bubble” or “honeycomb” appearance and scalloped margins in the case of the solid or multicystic variety. The unicystic type appears as a unilocular radiolucency often associated with the crown of an unerupted tooth (e.g., mandibular third molar); frequently, there is accompanying buccal and lingual cortical expansion. Histologic findings include combinations of cystic and solid features because of the tendency of these tumours to undergo cystic change.9 Several microscopic subtypes of ameloblastoma are recognized, although large tumours may show a combination of patterns.25 The patterns are: follicular, plexiform, acanthomatous, granular cell, desmoplastic, basal cell and various combinations of these.
Solid or Multicystic Ameloblastoma
This variant is observed in a wide age range of patients.26 Relatively uncommon in the first and second decades, its rate of occurrence is nearly equal in the third through seventh decades.27 There is no gender or racial predilection.
Radiographically, a multilocular radiolucency is most commonly observed, with or without resorption of adjacent tooth roots. Buccal and lingual cortical expansion frequently occurs and often results in perforation. Treatment of this variant generally requires an aggressive approach. The solid or multicystic ameloblastoma tends to penetrate between intact cancellous bony trabeculae at the tumour periphery before radiographic changes are evident. This results in a margin that lies beyond its apparent radiographic or clinical extent.28 Attempts to remove the tumour by curettage alone may leave behind small islands of tumour within bone, which later manifest as persistent or apparently recurrent disease.
Due to the highly infiltrative and aggressive nature of the solid or multicystic ameloblastoma, resection should include 1–1.5-cm linear bone margins.9 Furthermore, intraoperative specimen radiographs should be taken to confirm complete resection of the tumour. Soft tissue margins should involve sacrifice of one uninvolved anatomic barrier on the periphery of the specimen, according to anatomic barrier margin principles.29
Unicystic Ameloblastoma
This type of ameloblastoma is more commonly observed in younger patients, with most diagnosed in the second decade of life. More than 90% of these tumours are found in the mandible, usually in the molar–ramus region.30 Most, if not all, unicystic ameloblastomas present radiographically as a unilocular radiolucency not unlike a dentigerous cyst.4 There are 3 histological variants: luminal, intraluminal and mural. As the name suggests, in the luminal variant, the tumour is confined to the luminal surface of the cyst. The lesion consists of a fibrous cyst wall with a lining of ameloblastic epithelium. A unicystic ameloblastoma is classified as intraluminal when one or more nodules of the ameloblastoma projects from the cystic lining into the lumen of the cyst. Finally, a mural unicystic ameloblastoma is one in which tumour cells infiltrate the fibrous wall of the cyst to various depths.
Treatment and prognosis vary according to the histological variant. Treatment of a luminal or intraluminal variant of unicystic ameloblastoma by enucleation and curettage is often curative, with recurrence rate as low as 10–20%.31 However, certain circumstances dictate the need for resection, specifically recurrences (or tumour persistence), mural type and very large lesions with significant expansion. Tumours that recur after a well-performed enucleation and curettage are best approached with a more aggressive resection. Mural unicystic ameloblastomas are more aggressive than the luminal and intraluminal variants and, thus, require a surgical approach closer to that used for the solid or multicystic ameloblastoma. Very large lesions treated by enucleation and curettage can effectively result in a resection of the involved jaw or predispose the patient to subsequent pathologic fracture; in these cases, resection is indicated.
Peripheral Ameloblastoma
The rarest form of ameloblastoma is the peripheral or extraosseous type. These tumours present clinically as nonulcerated sessile or pedunculated gingival lesions and occur over a broad age range.9 Wide local excision is recommended for management of the peripheral ameloblastoma, and the cure rate is high when surgical margins are negative for tumour.32
When a large segment of mandibular bone has been resected, the choice between immediate and delayed reconstruction depends on the patient’s function and on cosmetic considerations. Advantages of immediate reconstruction of the mandible are an early return to masticatory function and minimal compromise of facial esthetics.33 Moreover, immediate reconstruction reduces the amount of surgical intervention required for the patient. On the other hand, delayed reconstruction of the mandible is associated with less risk of infection, dehiscence, and subsequent graft loss.34 The current gold standard for any method of mandibular reconstruction is to aim for a functional and esthetic outcome that includes the following33:
- Reproduction of the original contour, volume and projection of the mandible to duplicate pre-existing jaw relationships, mandibular movement and soft tissue support
- Primary fixation and stability of the hard tissue graft to the native jaw to facilitate rapid and optimal bone union
- Concurrent addressing of any associated soft tissue defects
- Measures to reduce or negate any motor or sensory neurologic defects
- Provision of dental implants to allow optimum restoration of dental function
Treatment Options
When the TMJ is to be reconstructed along with a large mandibular defect, surgeons must choose between autogenous bone grafting, alloplastic reconstruction, or a combination of these techniques.35 Autogenous options include free costochondral grafts12,13 and vascularized bone grafts.14,15 A viable alternative for mandibular reconstruction that avoids donor-site morbidity associated with harvesting free composite tissue is the combination of nonvascularized bone grafting with an alloplastic component. Since radiotherapy is not required after complete ablation of an ameloblastoma, a vascularized bone transfer is not necessary, which allows for immediate reconstruction with a prosthesis and nonvascularized graft.
Prosthetic reconstruction of the TMJ and associated mandibular defects (or anatomic abnormalities) may be achieved using a custom-made device. The Biomet prosthesis (Biomet Microfixation, Jacksonville, Florida) is a computer-assisted designed and manufactured device constructed on a stereolithic 3D model based on the patient’s own CT scan data. The fossa component consists of an ultra-high-molecular-weight polyethylene material, and the mandibular component is made of a cobalt–chromium alloy. A titanium plasma coating is sprayed on the undersurface of the mandibular prosthesis to facilitate osseointegration. These types of prostheses have been used for over 15-years with satisfactory long-term reliability and no documented foreign-body reactions or breakdown of materials.16
In the case reported above, a single surgery was performed for tumour ablation followed by autogenous bone grafting from the anterior iliac crest, and immediate reconstruction of the left TMJ. Because the prosthesis was rigidly fixated to the native mandible, postoperative maxillomandibular fixation (6–8 weeks) was not required; rather, the patient was functioning well within the first postoperative week. In addition, the custom-fabricated prosthesis resulted in a natural mandibular contour and avoided the extensive donor-site morbidity associated with other autogenous reconstructive methods, e.g., free fibula flap. The design of the prosthesis also allowed for simultaneous block grafting to facilitate complete rehabilitation with dental implants at a later time.
Conclusion
This case report demonstrates that reconstruction of the TMJ and large mandibular defects after benign tumour ablation can be satisfactorily accomplished using alloplastic components. In this case of surgical ablation of recurrent ameloblastoma, the result was a high degree of mandibular function and facial cosmesis with minimal donor-site morbidity and nearly immediate return to function. Performing all the steps in a single surgery resulted in a short recovery time for the patient, who was opening, closing and chewing within a week. Furthermore, coupling of the prosthesis with a nonvascularized bone graft facilitates future provision of dental implants to allow optimum restoration of dental function.
THE AUTHORS
| Gallery of all Figures in article | |||||

|

|
|
|
|
|
|

|

|
|
|
|
|
|

|
|
|
|
References
- Kramer IR, Pindborg JJ, Shear M. Histological typing of odontogenic tumours (International Histological Classification of Tumours), 2nd ed. Berlin: Springer-Verlag; 1992.
- Eckardt AM, Kokemüller H, Flemming P, Schultze A. Recurrent ameloblastoma following osseous reconstruction — a review of twenty years. J Craniomaxillofac Surg. 2009;37(1):36-41.
- Becelli R, Carboni A, Cerulli G, Perugini M, Iannetti G. Mandibular ameloblastoma: analysis of surgical treatment carried out in 60 patients between 1977 and 1998. J Craniofac Surg. 2002;13(3):395-400.
- Waldron CA. Odontogenic cysts and tumors. In: Neville BW, Damm DD, Allen CM, Bouquot JE, editors. Oral and Maxillofacial Pathology. 2nd ed. Philadelphia: WB Saunders; 2002. p. 589-642.
- Sehdev MK, Huvos AG, Strong EW, Gerold FP, Willis GW. Proceedings: ameloblastoma of maxilla and mandible. Cancer 1974;33(2):324-33.
- Gardner DG. Some current concepts on the pathology of ameloblastomas. Oral Surg Oral Med Oral Pathol Radiol Endod. 1996;82(6):660-9.
- Montoro JR, Tavares MG, Melo DH, Franco Rde L, Mello-Filho FV, Xavier SP, et al. Mandibular ameloblastoma treated by bone resection and immediate reconstruction. Braz J Otorhinolaryngol. 2008;74(1):155-7.
- Ghandhi D, Ayoub AF, Pogrel MA, MacDonald G, Brocklebank LM, Moos KF. Ameloblastoma: a surgeon’s dilemma. J Oral Maxillofac Surg. 2006;64(7):1010-4.
- Carlson ER. Odontogenic cysts and tumors. In: Miloro M, Gali GE, Larsen P, Waite P, editors. Peterson’s Principles of Oral and Maxillofacial Surgery. 3rd ed. Shelton, Conn.: People’s Medical Publishing House; 2012. p. 625-52.
- Reichart PA, Philipsen HP, Sonner S. Ameloblastoma: a biological profile of 3677 cases. Eur J Cancer B Oral Oncol. 1995;31B(2):86-99.
- Ueda M, Kaneda T. Combined chemotherapy and radiotherapy for advanced maxillary ameloblastoma: a case report. J Craniomaxillofac Surg. 1991;19(6):272-4.
- Lindqvist C, Pihakari A, Tasanen A, Hampf G. Autogenous costochondral grafts in temporo-mandibular joint arthroplasty. A survey of 66 arthroplasties in 60 patients. J Maxillofac Surg. 1986;14(3):143-9.
- Saeed NR, Kent JN. A retrospective study of the costocondral graft in TMJ reconstruction. Int J Oral Maxillofac Surg. 2003;32(6):606-9.
- Guyot L, Richard O, Layoun W, Cheynet F, Bellot-Samson V, Chossegros C, et al. Long-term radiological findings following reconstruction of the condyle with fibular free flaps. J Craniomaxillofac Surg. 2004;32(2):98-102.
- Thor A, Rojas RA, Hirsch JM. Functional reconstruction of the temporomandibular joint with a free fibular microvascular flap. Scand J Plast Reconstr Surg Hand Surg. 2008;42(5):233-40.
- Mercuri LG, Edibam NR, Giobbie-Hurder A. Fourteen-year follow-up of a patient-fitted total temporomandibular joint reconstruction system. J Oral Maxillofac Surg. 2007;65(6):1140-8.
- Feinberg SE, Steinberg B. Surgical management of ameloblastoma. Current status of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;81(4):383-8.
- Huffman GG, Thatcher JW. Ameloblastoma — the conservative surgical approach to treatment: report of four cases. J Oral Surg. 1974;32(11):850-4.
- Vedtofte P, Hjorting-Hansen E, Jensen BN, Roed-Peterson B. Conservative surgical treatment of mandibular ameloblastomas. Int J Oral Surg. 1978;7(3):156-61.
- Gardner DG, Pecak AM. The treatment of ameloblastoma based on pathologic and anatomic principles. Cancer. 1980;46(11):2514-9.
- Müller H, Slootweg PJ. The ameloblastoma, the controversial approach to therapy. J Maxillofac Surg. 1985;13(2):79-84.
- Gardner DG. A pathologist’s approach to the treatment of ameloblastoma. J Oral Maxillofac Surg. 1984;42(3):161-6.
- Black CC, Addante RR, Mohila CA. Intraosseous ameloblastoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;110(5):585-92.
- Carlson ER, Marx RE. The ameloblastoma: primary, curative surgical management. J Oral Maxillofac Surg. 2006;64(3):484-94.
- Mathew S, Rappaport K, Ali SZ, Busseniers AE, Rosenthal DL. Ameloblastoma. Cytologic findings and literature review. Acta Cytol. 1997;41(4):955-60.
- Ueno S, Nakamura S, Mushimoto K, Shirasu R. A clinicopathologic study of ameloblastoma. J Oral Maxillofac Surg. 1986;44(5):361-5.
- Takahashi K, Miyauchi K, Sato K. Treatment of ameloblastoma in children. Br J Oral Maxillofac Surg. 1998;36(6):453-6.
- Kramer IR. Ameloblastoma: a clinicopathological appraisal. Br J Oral Surg. 1963;1:13-28.
- Carlson ER. Pathologic facial asymmetries. Atlas Oral Maxillofac Surg Clin North Am. 1996;4(1):19-35.
- Gardner DG, Morton TH Jr, Worsham JC. Plexiform unicystic ameloblastoma of the maxilla. Oral Surg Oral Med Oral Pathol. 1987;63(2):221-3.
- Gardner DG, Corio RL. Plexiform unicystic ameloblastoma. A variant of ameloblastoma with a low-recurrence rate after enucleation. Cancer. 1984;53(8):1730-5.
- Woo SB, Smith-Williams JE, Sciubba JJ, Lipper S. Peripheral ameloblastoma of the buccal mucosa: case report and review of the English literature. Oral Surg Oral Med Oral Pathol. 1987;63(1):78-84.
- Baker A, McMahon J, Parmar S. Immediate reconstruction of continuity defects of the mandible after tumor surgery. J Oral Maxillofac Surg. 2001;59(11):1333-9.
- Lawson W, Loscalzo LJ, Baek SM, Biller HF, Krespi YP. Experience with immediate and delayed mandibular reconstruction. Larygoscope. 1982;92(1):5-10.
- Westermark A, Hedén P, Aagaard E, Cornelius CP. The use of TMJ Concepts prostheses to reconstruct patients with major temporomandibular joint and mandibular defects. Int J Oral Maxillofac Surg. 2011;40(5):487-96.