Abstract
Melanotic neuroectodermal tumour of infancy is a rare benign pigmented tumour that typically appears in the first year of life. We report an atypical presentation of this tumour, associated with an erupted primary tooth in a 7-month-old boy. We discuss the clinical, radiographic and histologic features of this rare tumour, as well as its surgical management and the follow-up treatment plan.
Melanotic neuroectodermal tumour of infancy (MNTI) is a rare benign pigmented tumour that most commonly presents in the first year of life. It is more predominant in boys1-3 and favors the premaxilla. It is also known as congenital melanocarcinoma, melanotic epithelial odontome, melanotic ameloblastoma, retinal anlage tumour, melanotic progonoma, pigmented adamantinoma, congenital pigmented epulis and melanocytoma. 1,4,5 Other known sites of this tumour include the mandible,2 brain,2,5,6 mediastinum,2,5 thigh,2 epididymis,1,2,5 foot2 and shoulder.2,5 Similar to other tumours of neuroectodermal origin, such as neuroblastoma and pheochromocytoma,5 MNTI is frequently associated with elevated urinary excretion of vanillylmandelic acid (VMA), a metabolite of epinephrine and norepinephrine.7 Although an increase in VMA is helpful, this symptom alone is not diagnostic of MNTI.5,7
The treatment of choice for this tumour is surgical excision. 1,8,9 Because of its relatively high recurrence rate (as high as 45%8,9), cases of MNTI should be monitored closely during the post-resection period and beyond. Although locally invasive, the risk of tumour metastasis is less than 5%.8,9 The benefits of adjuvant chemotherapy and radiation therapy to prevent recurrence of the tumour are unproven.1,8
Case History
An otherwise healthy 7-month-old boy was brought to the ambulatory dental clinic at BC Children's Hospital (BCCH) for investigation of swelling affecting the left maxillary alveolus. His pre- and perinatal histories were unremarkable. No history of trauma to the region was reported. Of interest, the infant's mother said that she had first noticed the eruption of the left primary central incisor at 2 months of age, and it was heralded by minor swelling in the area. The family physician had been consulted 3 weeks after the infant's mother noticed that the lesion had begun to expand rapidly. The physician prescribed a course of antibiotics based on a preliminary diagnosis of dental abscess. Three days later, when difficulties in feeding developed, he recommended that the infant be taken to the hospital emergency room. No airway difficulties were noted.
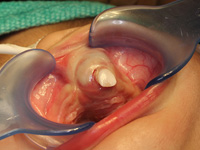
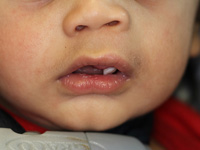
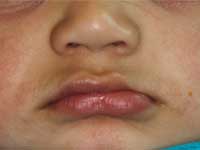
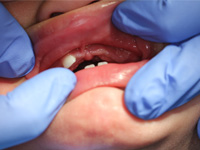
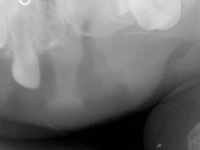
At the time of presentation in the dental department at BCCH, the infant appeared to be otherwise healthy and in no distress. Intraoral examination revealed a firm, smooth mass, 1.5 cm by 2 cm by 3 cm, in the anterior left maxilla (Fig. 1). Tooth 61 was the only tooth visible clinically; it was non-carious and extremely mobile within the enlarged mass. Expansion of the mass was primarily labial, although it was also palpable from the palatal aspect. Despite being nonerythematous, nonpurulent, nonpulsatile and immobile, the mass had a distinct bluish-purple tinge. Palpation of the mass appeared to be uncomfortable for the infant. Distortion of the upper lip and left nasal ala was noted, along with interference with mouth closing (Fig. 2). The tumour did not appear to cross the midline. Radiographic imaging of the premaxilla area revealed an expansile radiolucency with poorly defined margins. Displacement of the developing teeth 51, 62 and 61 within the radiolucency gave the teeth a "floating" appearance (Fig. 3). VMA testing was not carried out, as the infant's difficulty in feeding, the risk of progressive airway compromise and his parents' apprehension made the need for surgery urgent.
NOTE: Click to enlarge images.
Surgical Management and Histopathologic Findings
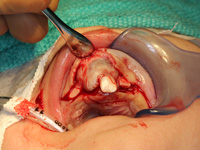
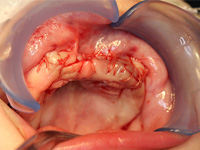
Excisional biopsy of the lesion was done under general anesthesia within hours of presentation. Fine-needle aspiration for fluid was negative. Sharp dissection of the lesion was followed by subperiosteal dissection, which revealed involvement of the vestibular and palatal cortices of the left maxillary alveolus. Both maxillary primary central incisors (erupted 61 and unerupted 51) and the unerupted left maxillary primary lateral incisor and canine (teeth 62 and 63) were excised with the lesion. Based on the clinical appearance of the tissues, the resected area was extended to include the presumed follicles of the maxillary permanent left central incisor, lateral incisor and canine (unerupted teeth 21, 22 and 23) to reduce the likelihood of the tumour's recurrence (Figs. 4, 5). Following resection, the soft tissue adjacent to the nasopalatine nerve was electrocauterized, and the surgical site was closed with a continuous vicryl suture (Fig. 6).
Gross examination of the tumour revealed a 2.8 cm by 1.7 cm by 1.6 cm specimen that was bilobular in outline and included some bone and pigmented soft tissue. The tumour appeared fairly well defined; however, microscopically, it was rather poorly delineated at the bony and soft tissue margin of resection. The tumour extended into the gingival soft tissues and intertrabecular spaces.
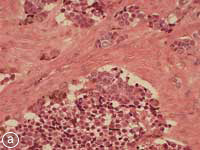
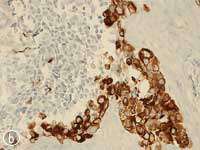
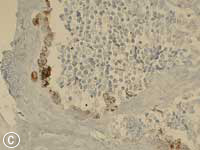
This MNTI exhibited a characteristic histologic appearance. It contained islands of cells separated by desmoplastic stroma (Fig. 7a). The islands included 2 types of cells: neuroendocrine cells, which have a less-differentiated, primitive appearance and rather scant non-distinct cytoplasm, and large epithelioid cells, which have a more complex, differentiated appearance. Melanin pigment may, at times, be identified in these epithelioid cells at the periphery of the islands. The less-differentiated cells were strongly positive for synaptophysin, an immunohistochemical marker confirming the neurosecretory features of this tumour. Staining of the larger advanced cells with cytokeratin and HMB-45 (human melanoma black 45) confirmed epithelial differentiation and features seen with melanocytic differentiation (Figs. 7b, c).
Figure 7: Histopathology slides of the tumour stained with (a) hematoxylin-eosin, (b) cytokeratin, (c) HMB-45.
Clinical Follow Up
The infant was re-examined at 1, 3 and 6 months after surgery, and no discrepancy in development or recurrence was evident clinically. All soft tissues had healed well and were within normal limits. Although the alveolar ridge was deficient at 1 month follow up, apparent improvement was noted by 3 months. At the 6 month follow-up appointment, the ridge deficiency appeared minimal and eruption of teeth 52, 71 and 81 were noted clinically (Figs. 8, 9). Radiographically, remodeling at the surgical site was apparent, with bone exhibiting the normal trabecular pattern (Fig. 10). The parents were reminded that long-term bi-annual follow up would be necessary to monitor for recurrence.
Discussion
The differential diagnosis for a rapid swelling in the premaxilla for this age group includes abscess, hemangioma,5 arteriovenous malformations,5 congenital epulis1,5 and neuroblastoma.1,9 Other anticipated entities may be rhabdomyosarcoma,1,9 Langerhans cell histiocytosis,5 Ewing's sarcoma,1,5,9 metastatic retinoblastoma,1 Wilms' tumour,9 fibrous dysplasia,5 lymphoma,1 teratoma1 and melanoma.1,9 In this case, the unique clinical presentation of the erupted primary tooth centred within the mass of the tumour made it difficult to distinguish from a dental abscess. However, the history, clinical progression and radiographic appearance were not consistent with this diagnosis and, therefore, biopsy was indicated.
Because of the urgent need for surgery, laboratory testing for urinary excretion of VMA was not done. It is important to note that the information from this test would not have altered the need for excisional biopsy, as there was an immediate need to address the infant's inability to feed adequately and the possibility of airway compromise.
It is plausible that, had the infant been referred for dental consultation at first presentation (based on the unusual circumstance of a neonatal tooth erupting in the maxilla), an earlier confirmation of diagnosis would have been possible. Prompt surgical intervention might have minimized the extent of resection and prevented the need to remove the developing follicles of the permanent incisors and ablation of the nasopalatine nerve.
Despite the benign nature of the tumour, microscopic evidence of tumour presence at the margin of resection indicated an increased likelihood of local recurrence and the possible need for more surgery.10
The impact of any residual surgical and alveolar growth-related deficit created by the loss of dentition in the maxillary alveolus will be re-evaluated periodically during this child's growth and development. However, definitive dental rehabilitation procedures may have to be delayed until growth is completed.
Conclusion
In summary, the importance of early detection and intervention in cases of MNTI cannot be overstated because of the rapidly destructive and invasive nature of this lesion. As pointed out in this case report, a number of known pathologic entities can present in the oral cavity of infants, but MNTI can be confirmed by its characteristic radiographic and histologic appearance. Any unusual oral findings that appear inconsistent with normal variation and reported history should be referred in a timely manner to a dental professional for assessment and definitive diagnosis.
It is likely that, because of the very early loss of permanent dentition and the high likelihood of tumour recurrence, final rehabilitation of this infant's dentition will require a long-term, multidisciplinary approach involving costly interventions.
THE AUTHORS
| Gallery of all Figures in article | |||||
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
References
- Bouckaert MM, Raubenheimer EJ. Gigantiform melanotic neuroectodermal tumor of infancy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;86(5):569-72.
- Cutler LS, Chaudhry AP, Topazian R. Melanotic neuroectodermal tumor of infancy: an ultrastructural study, literature review, and reevaluation. Cancer 1981;48(2):257-70.
- Choudhury M, Mukherjee J, Bajaj P, Jain A. Melanotic neuroectodermal tumor of infancy. Indian Pediatr. 1997;34(3):248-51.
- Pattanayak Monhanty S, Ray JG, Richa, Mukherjee S, Mandal C, Chaudhuri K. Melanotic neuroectodermal tumour of infancy. BMJ Case Rep. 2010 Nov 23.
- Selim H, Shaheen S, Barakat K, Selim AA. Melanotic neuroectodermal tumor of infancy: review of literature and case report. J Pediatr Surg. 2008;43(6):E25-9.
- Di Rocco F, Roujeau T, Pujet S, Peyre M, Zerah M. Slow-growing lambdoid melanotic neuroectodermal tumor of infancy. Eur J Pediatr. 2007;166(7):739-41.
- Rajappa S, Rao IS, Rao DR, et al. Melanotic progonoma – a case report and review of the literature. Ind J Med Paed Oncology. 2005;26(2):33-6.
- Shaia WT, Dinardo LJ, Underhill TE, Cesca CE. Recurrent melanotic neuroectodermal tumor of infancy. Am J Otolaryngol. 2002;23(4):249-52.
- Hamilton S, MacRae D, Agrawal S, Matic D. Melanotic neuroectodermal tumour of infancy. Can J Plast Surg. 2008;16(1):41-4.
- McGuire TP, Gomes PP, Forte V, Sándor GK. Rapidly growing intraoral swelling involving the maxilla of an infant. J Oral Maxillofac Surg. 2007;65(8):1595-9.





