View letters related to this article
Abstract
Pulp necrosis is an uncommon sequel to extrusive luxation in immature teeth with incomplete apical closure. In this report, we describe the management of severely extruded immature maxillary incisors and the outcome of revascularization to treat subsequent pulp necrosis. An 8.5-year-old boy with severe dentoalveolar trauma to the anterior maxillary region as a result of a fall was provided emergency treatment consisting of reduction of the dislodged labial cortical bone and repositioning of the central incisors, which had suffered extrusive luxation. When he presented with spontaneous pain involving the traumatized incisors a week later, the teeth were treated via a revascularization protocol using sodium hypochlorite irrigation followed by 3 weeks of intracanal calcium hydroxide, then a coronal seal of mineral trioxide aggregate and resin composite. Complete periradicular healing was observed after 3 months, followed by progressive thickening of the root walls and apical closure. Follow-up observations confirmed the efficacy of the regenerative treatment as a viable alternative to conventional apexification in endodontically involved, traumatized immature teeth.
Introduction
Extrusion is an injury characterized by partial axial displacement of a tooth.1 Clinically, the affected tooth appears elongated, is usually displaced in the palatal direction and demonstrates excessive mobility.2,3 Radiographically, extruded teeth appear to have an increased periodontal ligament space. Based on severance of the periodontal ligament that has not yet been exposed to desiccation or disarticulation of the tooth from the blood supply, Andreasen4 described extrusive luxation as “partial avulsion.” According to Lee and colleagues,3 this term is useful in terms of treatment approach, as the pulpal outcome of severe extrusion may be comparable to that of a replanted tooth.
The stage of apical development is a key factor in pulp healing after extrusive luxation.3,5,6 In teeth with open apices, the pulp has greater potential for healing, commonly followed by pulp canal obliteration; in patients with closed apices, the likelihood of pulp revascularization is low, usually leading to pulp necrosis.1,3,5,6 Once pulp necrosis is diagnosed, endodontic therapy should be initiated to eliminate infection and facilitate healing and retention of the tooth.3 If root development is incomplete, apexification is indicated to induce formation of a calcific barrier at the apex. However, this technique has several disadvantages, including up to 24 months of treatment, which often requires multiple visits and renewal of the intracanal dressing.7,8 Apical closure is unpredictable,9 and the tooth is susceptible to root fracture after prolonged exposure to calcium hydroxide (Ca(OH)2).10,11 Because of these concerns, the traditional Ca(OH)2-based apexification procedure has been modified by the introduction of an artificial apical barrier using mineral trioxide aggregate (MTA).12–15 Obturation of open apices with MTA plugs significantly reduces treatment time and results in favourable healing of periradicular tissues.12,14,16,17 However, MTA plugs cannot stimulate physiologic apical closure and thickening of radicular dentin, leaving the tooth’s structural integrity compromised.18,19
Revascularization is an emerging regenerative endodontic treatment approach that aims to allow continuation of root development and tissue regeneration in immature necrotic teeth.20,21 The root canal is disinfected with sodium hypochlorite, followed by placement of an intracanal medicament, such as calcium hydroxide or a combination of ciprofloxacin, metronidazole and minocycline.22 After disinfection, the antibiotic paste is removed and apical bleeding is induced to form a blood clot below the coronal level. The root canal orifice is then sealed with MTA, and the tooth crown is restored permanently.
This protocol has been successful, as evidenced by increased root length, thickening of the root walls and apical closure of varying degrees.23–28 In the following case, we describe the management of severely extruded immature maxillary incisors and the outcome of revascularization in the treatment of pulp necrosis subsequent to the trauma.
Case Report
A healthy 8.5-year-old boy was admitted to the pediatric dentistry clinic 6 hours after a fall in his schoolyard. Reportedly, an emergency examination had been carried out by a hospital pediatrician, who found the patient to be free of neurologic and general physical symptoms and referred him for management of dentoalveolar trauma.
The child was unable to close his mouth or speak properly because of severely displaced maxillary central incisors, evident on extraoral view (Fig. 1a). Intraoral examination showed severe extrusive luxation of the incisors along with a fractured labial cortical bone (Fig. 1b). The teeth were excessively mobile and the maxillary right central incisor showed pronounced displacement in the palatal direction. The palatal segment of the alveolar bone was slightly mobile on palpation, but did not appear to be dislodged. The neighbouring lateral incisors displayed normal mobility. The attached gingiva distal to the right lateral incisor was lacerated (Fig. 1b). A periapical radiograph revealed increased apical periodontal ligament space in both incisors, along with palatal displacement of the right central incisor (Fig. 1c). In both teeth, root development was incomplete, and wide root canals and open apices were evident.
Following removal of the blood clot with copious saline irrigation (Fig. 1d), the dislodged buccal cortical bone was gently repositioned. The extruded incisors were then meticulously repositioned by conventional digital maneuver, with no sign of resistance caused by a clot blockage. A semi-rigid splint made of 0.9-mm monofilament fishing line was bonded to the lateral and central incisors using acid-etch composite resin (Fig. 1e). After suturing of soft tissue lacerations, a radiograph was taken to confirm correct reduction and repositioning (Fig. 1f). The patient was prescribed amoxicillin and ibuprofen, and scheduled for a follow-up visit.
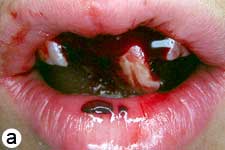
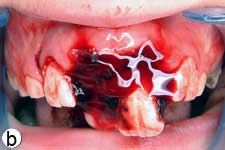
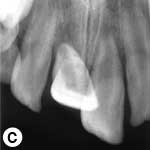
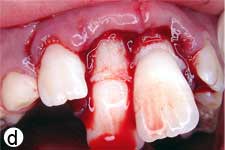
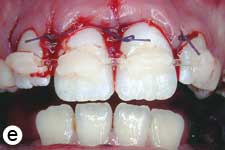
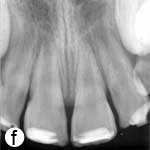
Figure 1: Initial examination of patient. a) Extraoral view, demonstrating the extent of jaw closure; b) intraoral and c) radiographic views of extruded incisors; d) intraoral view following removal of the blood clot with saline irrigation; e) view of the incisors after reduction, splinting and suturing; f) radiographic view of the incisors after repositioning, revealing the wide root canals and open apices.
A week later, the patient returned with severe spontaneous pain involving the traumatized incisors. The teeth were tender on palpation, and radiographic examination revealed periapical radiolucency. Because of the patient’s incomplete root development and wide open apices, traditional endodontic therapy using Ca(OH)2-based apexification or placement of an apical barrier with MTA would seriously compromise the structural integrity of the tooth. Therefore, regenerative endodontic treatment of the affected incisors was considered. After comprehensive discussion of the risks and possible outcomes of this treatment and the treatment plan in case of failure, the consent of the patient and parents was obtained and treatment was initiated at the same visit.
After anesthesia, the pulp chambers were accessed. Isolation was achieved using cotton rolls and gauze, as a rubber dam could not be placed in the presence of the trauma splint. Each root canal orifice was gently irrigated with 10 mL of 2.5% sodium hypochlorite (NaOCl) without instrumentation. Ca(OH)2 powder (Merck, Darmstadt, Germany) was mixed with sterile saline in a 3:1 ratio to produce a thick, homogeneous paste. The mixture was placed in the pulp chamber using a plastic carrier and loosely packed into the coronal portion of the root canals with moist cotton pellets. Finally, the access cavity was sealed with Cavit (3M ESPE, Seefeld, Germany) (Fig. 2a). A week later, the patient was recalled for removal of the trauma splint and, 3 weeks later, for evaluation of the intracanal medication.
After 3 weeks, both teeth were asymptomatic. They were anesthetized using 2% mepivacaine (Citanest, AstraZeneca, UK) without a vasoconstrictor, isolated with a rubber dam and reaccessed. The Ca(OH)2 paste was removed with copious 2.5% NaOCl irrigation, and the root canals received a final irrigation with 10 mL sterile saline and were dried. Apical bleeding was induced by gentle irritation using size 15 K-files. After a blood clot had formed, MTA (Dentsply Tulsa Dental, Tulsa, OK) was prepared according to the manufacturer’s instructions and gently adapted over the blood clot. A wet cotton pellet was placed over the MTA, and the access cavity was temporarily restored with conventional glass ionomer cement. Final resin composite restorations were placed 1 week later (Fig. 2b), and the patient was scheduled for regular follow-up visits.
The teeth remained asymptomatic during the 18-month evaluation period. At 3 months, the teeth showed complete periapical healing and, thereafter, root development and closure of the apices continued (Fig. 2c).
To quantify the increase in root width and length, the radiographs obtained immediately after treatment and 18 months later were converted to 32-bit TIFF files using ImageJ analysis program (v.1.44p, National Institutes of Health, Bethesda, MD). The TurboReg plug-in (Biomedical Imaging Group, Swiss Federal Institute of Technology, Lausanne, Switzerland)29 was used to mathematically align the two images as described by Bose and colleagues.28 Because the 18-month radiograph showed less distortion, it was used as the “source” image, while the postoperative radiograph, which required correction, was used as the “target” image.28 Following alignment of the images using TurboReg (Fig. 2d), a scale was added, and root lengths and root wall thicknesses were measured.28 This revealed an increase of 18.16% and 17.14% in the root lengths and 40.54% and 75.64% in the root widths of the right and left incisors, respectively.
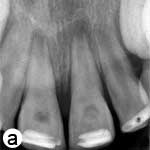
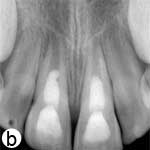
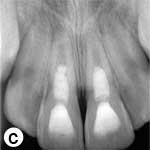
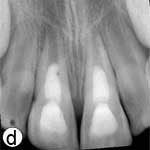
Figure 2: a) Radiographic view of the teeth after intracanal application of calcium hydroxide (Ca(OH)2) paste; periradicular radiolucencies are evident in both roots. b) Periapical radiograph showing the coronal mineral trioxide aggregate (MTA) barrier and final composite restoration. c) Radiographic view at 18 months follow-up, demonstrating narrowing of root canal in the apical third and thickening of the lateral walls. A normal bony architecture at the periradicular region is evident. d) Image b after correction (alignment) with ImageJ and the TurboReg plugin using c as the “source” image for mathematical correction.
At 12 months, a positive response to a cold test was first observed, but the response of both teeth to electric pulp testing (EPT) was inconsistent. At 18 months, response to cold testing was still positive and both teeth showed a consistent, delayed response to EPT. The patient has been attending regular follow-up appointments; his teeth have remained asymptomatic, with normal mobility and gingiva in good condition.
Discussion
Pulp necrosis is a relatively uncommon sequel to extrusive luxation in immature teeth with wide-open apices,5 because of the high likelihood of revascularization and subsequent root development in these teeth. However, the risk increases significantly in the case of severe extrusion3 and, if pulp necrosis occurs, it is likely to be an early event.3,5,30
Regenerative endodontic techniques may enhance continued root development21 and, therefore, offer an alternative approach to the management of traumatized immature permanent teeth with pulp necrosis and periradicular infection.24,31 A growing body of evidence supports the possibility of residual viable pulpal tissue in the wide root canal or apical region of necrotic immature teeth, which may survive the infection and allow continued apical development.25,32,33 Stem cells from the apical papilla may also survive infection, because of their proximity to the periapical tissues.26,32,33 Following proper endodontic disinfection, these cells may differentiate under the influence of surviving epithelial cells of Hertwig’s root sheath and initiate continued root development.26,33 Once the regenerative process is induced, the presence of a wide apical foramen and root canal enhances the ingrowth of small blood vessels and regenerated tissues.26
In the revascularization protocol, infected root canals should be treated as conservatively as possible.20,25,31 This is best achieved by copious irrigation with 2.5%–5.25% NaOCl and no instrumentation. At the same appointment, intracanal medication is put in place to disinfect the root canal and left for 3–4 weeks. Previous reports have demonstrated the effectiveness of a triple antibiotic paste consisting of metronidazole, ciprofloxacin and minocycline in the disinfection of infected root canals,22,34 including those of immature teeth with apical periodontitis.25,35 The main disadvantage of this paste is minocycline-induced crown discoloration,36,37 which might be reduced, but not prevented by prior sealing of the coronal dentin with bonding agents.37
Ca(OH)2 has also been used successfully for disinfection of root canals before revascularization.23,26,28 Bose and colleagues28 showed that placement of Ca(OH)2 in the coronal half of the root canal contributed to a significant increase in root length and wall thickness, comparable to that achieved with the triple antibiotic paste.
In the current case, the teeth were asymptomatic after treatment with Ca(OH)2: continuing root development was observed, symptoms of infection were absent and no crown discoloration occurred. In a retrospective study, Chueh and colleagues26 showed a high rate of progressive calcification of the root canal space in teeth medicated with Ca(OH)2, suggesting that root development induced by regenerative endodontic treatment may not follow a natural pattern. Thus, despite the absence of root canal obliteration in the current case, progressive calcification may occur in the longer run.
Previous studies of the revascularization procedure in traumatized, immature incisors have reported a lack of sensitivity to both cold testing and EPT.24,30,38 In the absence of histologic data from humans, the reasons for both positive and negative responses to thermal and electrical stimuli should be interpreted with caution, as lack of response could merely be a result of the thickness of the MTA and restorative materials preventing stimulation of vital tissues within the root canal.39 The use of a collagen matrix to control the thickness of the coronal MTA barrier30 and placement of the MTA barrier close to the cementoenamel level39 might increase the likelihood of a positive response, provided that the regenerated tissue in the root canal contains nerves. Based on these considerations, the inconsistent responses of the extruded incisors to EPT in contrast to cold testing might have resulted from the thick MTA barriers, which occupied almost half the length of the root canal.
The favourable short-term results in this case of severe extrusive luxation show that regenerative endodontic treatment of pulpally involved traumatized immature teeth is a viable alternative to apexification or artificial apical barrier techniques. Although the nature of the regenerated tissue within the root canal is yet to be elucidated in humans, it is evident that this technique can allow for continued root development and apical closure. More clinical data is required to confirm the predictability of this approach.
THE AUTHORS
References
- Andreasen JO, Andreasen FM, Bakland LK, Flores MT. Traumatic dental injuries: a manual. 1st ed. Copenhagen: Munksgaard; 2000. p. 34-5.
- Andreasen FM Andreasen JO. Luxation injuries. In: Andreasen JO, Andreasen FM, editors. Textbook and color atlas of traumatic injuries to the teeth. 3rd ed. Copenhagen: Monksgaard; 1994. p. 318.
- Lee R, Barrett EJ, Kenny DJ. Clinical outcomes for permanent incisor luxations in a pediatric population. II. Extrusions. Dent Traumatol. 2003;19(5):274-9.
- Andreasen JO. Luxation of permanent teeth due to trauma. A clinical and radiographic follow-up study of 189 injured teeth. Scand J Dent Res. 1970;78(3):273-86.
- Andreasen FM, Pedersen BV. Prognosis of luxated permanent teeth — the development of pulp necrosis. Endod Dent Traumatol. 1985;1(6):207-20.
- Andreasen FM, Zhijie Y, Thomsen BL. Relationship between pulp dimensions and development of pulp necrosis after luxation injuries in the permanent dentition. Endod Dent Traumatol. 1986;2(3):90-8.
- Cvek M. Prognosis of luxated non-vital incisors treated with calcium hydroxide and filled with gutta-percha. A retrospective clinical study. Endod Dent Traumatol. 1992;8(2):45-55.
- Abbot PV. Apexification with calcium hydroxide — when should the dressing be changed? The case for regular dressing changes. Aust Endod J. 1998;24(1):27-32.
- Rafter M. Apexification: a review. Dent Traumatol. 2005;21(1):1-8.
- Andreasen JO, Farik B, Munksgaard EC. Long-term calcium hydroxide as a root canal dressing may increase risk of root fracture. Dent Traumatol. 2002;18(3):134-7.
- Doyon GE, Dumsha T, von Fraunhofer JA. Fracture resistance of human root dentin exposed to intracanal calcium hydroxide. J Endod. 2005;31(12):895-7.
- Mente J, Hage N, Pfefferle T, Koch JM, Dreyhaupt J, Staehle HJ, et al. Mineral trioxide aggregate apical plugs in teeth with open apical foramina: a retrospective analysis of treatment outcome. J Endod. 2009;35(10):1354-8.
- Simon S, Rilliard F, Berdal A, Machtou P. The use of mineral trioxide aggregate in one-visit apexification treatment: a prospective study. Int Endod J. 2007;40(3):186-97.
- Holden DT, Schwartz SA, Kirkpatrick TC, Schindler WG. Clinical outcomes of artificial root-end barriers with mineral trioxide aggregate in teeth with immature apices. J Endod. 2008;34(7):812-7.
- Witherspoon DE, Small JC, Regan JD, Nunn M. Retrospective analysis of open apex teeth obturated with mineral trioxide aggregate. J Endod. 2008;34(10):1171-6. Epub 2008 Aug 13.
- Sarris S, Tahmassebi JF, Duggal MS, Cross IA. A clinical evaluation of mineral trioxide aggregate for root-end closure of non-vital immature permanent incisors in children — a pilot study. Dent Traumatol. 2008;24(1):79-85.
- Cehreli ZC, Sara S, Uysal S, Turgut MD. MTA apical plugs in the treatment of traumatized immature teeth with large periapical lesions. Dent Traumatol. 2011;27(1):59-62. Epub 2010 Dec 5.
- Huang GT. Apexification: the beginning of its end. Int Endod J. 2009;42(10):855-66. Epub 2009 June 22.
- Wang X, Thibodeau B, Trope M, Lin LM, Huang GT. Histologic characterization of regenerated tissues in canal space after the revitalization/revascularization procedure of immature dog teeth with apical periodontitis. J Endod. 2010;36(1):56-63.
- Banchs F, Trope M. Revascularization of immature permanent teeth with apical periodontitis: new treatment protocol? J Endod. 2004;30(4):196-200.
- Murray PE, Garcia-Godoy F, Hargreaves KM. Regenerative endodontics: a review of current status and a call for action. J Endod. 2007;33(4):377-90. Epub 2007 Feb 20.
- Hoshino E, Kurihara-Ando N, Sato I, Uematsu H, Sato M, Kota K, et al. In-vitro antibacterial susceptibility of bacteria taken from infected root dentine to a mixture of ciprofloxacin, metronidazole and minocycline. Int Endod J. 1996;29(2):125-30.
- Chueh LH, Huang GT. Immature teeth with periradicular periodontitis or abscess undergoing apexogenesis: a paradigm shift. J Endod. 2006;32(12):1205-13. Epub 2006 Oct 13.
- Cotti E, Mereu M, Lusso D. Regenerative treatment of an immature, traumatized tooth with apical periodontitis: report of a case. J Endod. 2008;34(5):611-6.
- Jung IY, Lee SJ, Hargreaves KM. Biologically based treatment of immature permanent teeth with pulpal necrosis: a case series. J Endod. 2008;34(7):876-87. Epub 2008 May 16.
- Chueh LH, Ho YC, Kuo TC, Lai WH, Chen YH, Chiang CP. Regenerative endodontic treatment for necrotic immature permanent teeth. J Endod. 2009;35(2):160-4. Epub 2008 Dec 12.
- Ding RY, Cheung GS, Chen J, Yin XZ, Wang QQ, Zhang CF. Pulp revascularization of immature teeth with apical periodontitis: a clinical study. J Endod. 2009;35(5):745-9.
- Bose R, Nummikoski P, Hargreaves K. A retrospective evaluation of radiographic outcomes in immature teeth with necrotic root canal systems treated with regenerative endodontic procedures. J Endod. 2009;35(10):1343-9. Epub 2009 Aug 15.
- Thévenaz P, Ruttimann UE, Unser M. A pyramid approach to subpixel registration based on intensity. IEEE Trans Image Process. 1998;7(1):27-41.
- Jacobsen I. Criteria for diagnosis of pulp necrosis in traumatized permanent incisors. Scand J Dent Res. 1980;88(4):306-12.
- Petrino JA, Boda KK, Shambarger S, Bowles WR, McClanahan SB. Challenges in regenerative endodontics: a case series. J Endod. 2010;36(3):536-41. Epub 2009 Dec 6.
- Sonoyama W, Liu Y, Yamaza T, Tuan RS, Wang S, Shi S, et al. Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: a pilot study. J Endod. 2008;34(2):166-71.
- Huang GT, Sonoyama W, Liu Y, Liu H, Wang S, Shi S. The hidden treasure in apical papilla: the potential role in pulp/dentin regeneration and bioroot engineering. J Endod. 2008;34(6):645–51.
- Sato I, Ando-Kurihara N, Kota K, Iwaku M, Hoshino E. Sterilization of infected root-canal dentine by topical application of a mixture of ciprofloxacin, metronidazole and minocycline in situ. Int Endod J. 1996;29(2):118-24.
- Windley W 3rd, Teixeira F, Levin L, Sigurdsson A, Trope M. Disinfection of immature teeth with a triple antibiotic paste. J Endod. 2005;31(6):439-43.
- Reynolds K, Johnson JD, Cohenca N. Pulp revascularization of necrotic bilateral bicuspids using a modified novel technique to eliminate potential coronal discolouration: a case report. Int Endod J. 2009;42(1):84-92.
- Kim JH, Kim Y, Shin SJ, Park JW, Jung IY. Tooth discoloration of immature permanent incisor associated with triple antibiotic therapy: a case report. J Endod. 2010;36(6):1086-91.
- Thibodeau B. Case report: pulp revascularization of a necrotic, infected, immature, permanent tooth. Pediatr Dent. 2009;31(2):145-8.
- Torabinejad M, Turman M. Revitalization of tooth with necrotic pulp and open apex by using platelet-rich plasma: a case report. J Endod. 2011;37(2):265-8.
